SandboxPKA
From Proteopedia
The c-Abl protein 1 (ABL1), also known as Abelson kinase, is a non-receptor tyrosine kinase that plays a role in many key processes linked to cell growth and survival such as cytoskeleton remodeling in response to extracellular stimuli, cell motility and adhesion, receptor endocytosis, autophagy, DNA damage response and apoptosis. [1] [2] Activity of c-Abl protein is negatively regulated by its SH3 domain, and deletion of the SH3 domain turns ABL1 into an oncogene. In more than 90% cases, chronic myeloid leukemia (CML) is caused by a chromosomal abnormality that results in the formation of the Philadelphia chromosome. This chromosome is formed by fusion between Abelson (Abl) tyrosine kinase gene at chromosome 9 and break point cluster region (BCR) gene at chromosome 22, resulting in the chimeric oncogene BCR-Abl and a constitutively active BCR-Abl tyrosine kinase. The Bcr-Abl pathway has many downstream pathways including the Ras/MAPK pathway, which leads to increased proliferation due to increased growth factor-independent cell growth. It also affects the Src/Pax/Fak/Rac pathway. This affects the cytoskeleton, which leads to increased cell motility and decreased adhesion. The PI/PI3K/AKT/BCL-2 pathway is also affected. The last pathway that Bcr-Abl affects is the JAK/STAT pathway, which is responsible for proliferation.
Small molecule inhibitors of BCR-Abl that bind to the kinase domain can be used to treat CML [3]
Contents |
From the Sequence to the Structure
All of the protein kinases have a similar bilobal fold, and their key structural features have been well studied. Like others, the abelson kinase incorporates a highly conserved bi-lobed structure with an adenosine triphosphate (ATP) binding domain situated in a deep cleft between the N- and C-terminal lobes. Adjacent to this is the centrally located activation loop that incorporates a conserved Asp-Phe-Gly (DFG) sequence and controls catalytic activity by switching between different states in a phosphorylation-dependent manner [4].
The N-terminal half of the protein includes an N-terminal “cap” of 80 residues that is important for autoinhibition, followed by an SH3 domain, an SH2 domain, and a tyrosine kinase domain. The C-terminal half of c-Abl includes binding elements for SH3 domains, nuclear localization and export signals, a DNA binding functionality, and an actin binding domain. Human cells express two splice variants of c-Abl, Abl 1a and Abl 1b, which only differ in the far N-terminal region. Abl 1b is myristoylated, whereas Abl1a is not.
The fusion of the gene encoding c-Abl with the breakpoint cluster region (BCR) gene, results in the formation of a fusion protein, BCR-Abl, in which the entire c-Abl protein is preserved without mutation, except for the “cap” region upstream of the SH3 domain.
| |||||||||||
Catalytic Reaction
Protein kinases are a group of enzymes that possess a catalytic subunit that transfers the gamma (terminal) phosphate from nucleotide triphosphates (often ATP) to one or more amino acid residues in a protein substrate side chain, resulting in a conformational change affecting protein function.
The kinases are classified into several broad groups depending on their substrate specificity. c-Abl is included in the group of Tyrosine kinases: [8]
Regulatory spine
There is a highly conserved spatial motif that was found in every active kinase, but missing in inactive kinases. This motif comprises four non-consecutive hydrophobic residues, two from the N-lobe and two from the C-lobe. It cannot be identified from the primary sequence, and the roles of these four residues had never been considered in previous analysis of protein kinase structure and function. Because the middle part of this motif, the C-helix and the activation loop, can be very mobile, the hydrophobic spine can be dynamically assembled or disassembled, thereby regulating protein kinase activity. In this R-spine we can find a backbone of the His/Tyr, anchored to the F-helix which serves as the base of the R-Spine.
Catalytic spine
Like the R-spine, it comprises residues from both lobes; however, this spine is completed by the adenine ring of ATP. It was thus termed as the catalytic (C) spine. The two C-spine residues in the N-Lobe, Val in β2 and Ala from the ‘‘AxK’’ motif in β3, are docked directly onto the adenine ring of ATP, whereas in the C-lobe it is Leu that docks directly onto the adenine ring. Leu residue lies in the middle of β7. Identification of the C-spine shows that this helix contributes to the positioning of ATP with respect to the rigid hydrophobic core of the C-lobe.
Bcr-Abl tyrosine kinase autoregulation
The human Bcr protein contains 1,271 amino acids and multiple domains, including the oligomerization domain (OLI), the serine/threonine kinase domain (S/TK), the domain homologous to the human Dbl and yeast Cdc24 proteins (DH) and the domain with GTPase-activating activity for Rac (RacGAP). The c-Abl protein contains 1,097 amino acids, which include the Src-homology domains 3/2 (SH3/SH2), tyrosine kinase domain (TK), nuclear translocalization signal (NTS), DNA binding domain (DB) and actin-binding motif (AB). Two domains are essential for transforming activity — OLI from Bcr and TK from Abl. Depending on the site of the breakpoint in the Bcr gene, the fusion protein can vary in size from 190 to 230 kDa. Each fusion protein contains the same portion of the c-Abl protein but differs in the length of the Bcr portion. [9]
Normal ABL has a tri-dimensional structure which is tightly preserved in a closed, inactive conformation order to prevent oncogenic activation. The maintenance of this inactive conformation is possible by:
1- The "latching" of the myristoylated N-terminal sequence which is directly linked to a myristoyl recognition sequence on the c-lobe of the SH1 kinase domain,
2- The close contact between SH3 and SH2 domain,
3- The interactions between SH3 domain and the C-lobe of the kinase domain. These interactions clamp the structure and prevent the kinase to switch to an active conformation, a process which requires the phosphorylation of Tyr 412 residue and the "unlatching" of the myristoyl group from the C-Lobe of the kinase domain. The attachment of proline-rich SH2 and SH3 ligands leads to the complete switch of the protein to an open, active conformation of the kinase. The N-terminal myristoylation (autoregulatory role) is deleted during the t(9;22) translocation. [10]
Bcr-Abl tyrosine-kinase inhibitors
| |||||||||
| Human Abl kinase domain in complex with imatinib | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Gene: | ABL1 (Homo sapiens) | ||||||||
| Activity: | Non-specific protein-tyrosine kinase, with EC number 2.7.10.2 | ||||||||
| Related: | 1iep, 1fpu | ||||||||
| |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
1st Generation TKIs: Gleevec
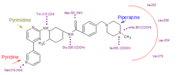
Imatinib mesylate (STI571 or Gleevec), discovered in 1992, is a Bcr-Abl tyrosine kinase inhibitor (Bcr-Abl TKI) with a high affinity for the ABL kinase. Imatinib is the most common first generation drug used for the CML treatment. Imatinib achieves BCR-ABL kinase inhibition by binding to the inactive, unphosphorylated conformation of the kinase (DFG-out) , thereby reducing the availability of the catalytically active phosphorylated conformation (DFG-in), necessary for ATP binding. This blocks signal transduction, ultimately resulting in inhibition of proliferation and loss of viability.[11]. Imatinib also inhibits the Abl protein of non-cancer cells but cells normally have additional redundant tyrosine kinases which allow them to continue to function even if Abl is inhibited.
Imatinib (a 2-phenylaminopyrimidine derivative) is used for treating CML, gastrointestinal stromal tumours and other diseases. By 2011, Gleevec had been FDA approved to treat ten different cancers.
Pharmacokinetics: Imatinib is rapidly absorbed when given orally, and is highly bioavailable: 98% of an oral dose reaches the bloodstream. Metabolism of imatinib occurs in the liver and is mediated by several isozymes of the cytochrome P450 system. The main metabolite, N-demethylated piperazine derivative, is also active. The half-lives of imatinib and its main metabolite are 18 and 40 hours, respectively. [12]
Adverse effects: The most common side effects include weight gain, reduced number of blood cells (neutropenia, thrombocytopenia, anemia), headache, edema, nausea, rash, and musculoskeletal pain.
Second generation TKIs: Nilotinib, Dasatinib
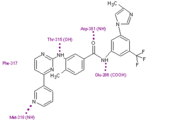
Nilotinib
Nilotinib (AMN107) is a second-generation oral TKI. It was designed based on the crystal structure of imatinib to be highly active against a wide range of imatinib-resistant BCR-ABL mutants and is approved for the treatment of newly diagnosed or imatinib-resistant or -intolerant CML. Nilotinib was designed to maintain binding to the inactive conformation of the ABL kinase domain, while incorporating alternative binding groups to the N-methylpiperazine moiety and preserving an amide pharmacophore to retain H-bond interactions with Glu286 and Asp381. [13] Substitution of the N-methylpiperazine moiety present in imatinib by a phenyl group bearing trifluoromethyl and imidazole substituents in the nilotinib structure greatly contributes to the potency of nilotinib by reducing the requirement for hydrogen bonding with nilotinib (four H-bond interactions compared with six H-bonds for imatinib). Among patients with imatinib resistant CML, nilotinib has not been associated with the toxic effects commonly seen with imatinib treatment, such as fluid retention, edema, and weight gain [14]
Dasatinib
Dasatinib is also a second-generation TKI. Dasatinib binds kinases of the SCR family (0,2 nM<IC50<1,1nM). It binds both the active and inactiv forms of the Abl kinase with a higher affinity (300 times) than Imatinib. Dasatinib is active against most BCR-ABL mutants with the exception of T315I. [15] Dasatinib is well tolerated, but adverse effects can include muscle and joint aches, fatigue, dermatologic complaints such as rash and acne, headaches, and diarrhea [16]
Third generation drugs
New mutations of Bcr-Abl tyrosine kinase are still being discovered and some old go unconquered. To overcome those mutations, new generation drugs are being developed to treat patients with CML.
Bosutinib (SKI-606)
Bosutinib has been seen to be active in chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. [17] On September 4, 2012, the U. S. Food and Drug Administration approved bosutinib tablets (Bosulif, Pfizer, Inc.) for the treatment of chronic, accelerated, or blast phase Philadelphia chromosome positive (Ph+) CML in adult patients with resistance or intolerance to prior therapy. [18]
is based on a quinoline scaffold and is structurally related to the AstraZeneca quinazoline template. [19]
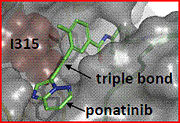
Ponatinib (AP24534)
Ponatinib was identified using structure base drug design and focused synthetic libraries of trisubstituted purine analogs. It can inhibit, on nanomolar scale, Src and Bcr-Abl kinases including many common imatinib resistant Bcr-Abl mutations, like T315I mutation. The key structural feature of the molecule is a carbon-carbon triple bond linkage that makes productive hydrophobic contact with the side chain of I315, allowing inhibition of the T315I mutant. The triple bond also acts as an inflexible connector that enforces correct positioning of the two binding segments of AP24534 into their established binding pockets. AP24534 maintains an extensive hydrogen-bonding network and occupies a region of the kinase that overlaps significantly with the imatinib binding site. [20]
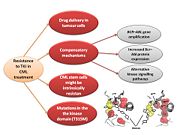
Resistance
Resistance to first generation TKI (Imatinib)
In the majority of cases, resistance is caused by reactivation of Bcr–Abl kinase activity. There are several mechanisms currently known that may result in Imatinib resistance [21]:
1. Plasma protein binding: Imatinib binds very strongly to the ∂-1-acid glycoprotein 1. Changes in ∂-1-acid glycoprotein may change the amount of binding of this drug, thus changing drug availability. This process can be also classified as a pharmacokinetic parameter.
2. Drug efflux: Imatinib is a substrate for the P-glycoprotein pump, which can result in decreased intracellular concentrations of Imatinib.
3. T315I mutation: In the presence of Imatinib, cells that generate mutations in BCR-ABL can overcome the ability of this drug to inhibit cell division. Mutations that alter the Imatinib binding site without affecting the adenosine triphosphate binding site or the active site of the kinase are very effective at inducing drug resistance. The gatekeeper in c-Abl kinase is a smaller threonine (Thr 315) that is not an effective stabilizer of the R-spine, but mutation in this residue is the most common mechanism implicated in secondary drug resistance. Usually, the gatekeeper is substituted by isoleucine or methionine and avoids Gleevec entrance to ATP-binding domain.
4. P-loop mutation: the structure of Bcr-Abl contains two flexible loops, the ATP-binding P-loop and the activation loop. Mutations in these loops destabilize arrangement of the loops such that the kinase domain cannot assume the inactive conformation required for Imatinib binding. There are clinical data indicating that Bcr-Abl mutations in the P-loop is 70-100 fold less sensitive to imatinib compared with native Bcr-Abl. [22]
5. Activation of different kinases: although the 9:22 translocation is necessary to initiate CML, BCR-ABL is only one of several kinases capable of maintaining the proliferation rate of the cell while inhibiting apoptosis.
6. Gene amplification: as the number of Philadelphia chromosomes increases, the number of BCR-ABL proteins expressed in the cell increases and the efficacy of Imatinib decreases.
7. Inadequate plasma levels of Imatinib: Subtherapeutic dosing is highly likely to result in the selection of a resistant clone. That’s why measurement of plasma levels is very important. To solve this problem second generation drugs (Nilotinib) were developed.
8. Pharmacokinetic parameters: The cytochrome P450 enzymes metabolize Imatinib by the CYP3A4 isoform. The variability in CYP3A4 activity among individuals is may contribute to some of the variation in imatinib levels between patients. In addition, other drugs taken by patients can alter CYP3A4 activity. Further polymorphism studies are mandated to understand the significance of cytochrome P450 enzymes on the plasma levels of Imatinib and response to therapy in CML patients.
Resistance to second generation of TKI (Nilotinib/Dasatinib)
Nilotinib and Desatinib are ineffectiveness against the T315I mutant. It is important to underline that all mutations except T315I were effectively suppressed by increasing Nilotinib concentration. Although Dasatinib is much more potent than Imatinib it is possible, like with Nilotinib, that its specific mode of binding to Abl may lead to new vulnerable sites that could confer new kinds of drug resistance. Mutations have been found on Phe317 so that is a potential vulnerable site for this drug [23]
Resistance to drugleads
T315 mutation is resistant to Bosutinib. In contrast to imatinib, nilotinib and dasatinib, bosutinib is not an efficient substrate for multidrug resistance (MDR) transporters that promotes efflux of foreign molecules from cells. Bosutinib even inhibits these transporter proteins in higher concentrations. [24]
New strategies in controlling drug resistance
Dealing with the rise of these resistant clones has presented an important challenge to health care providers. Thus, new therapeutic strategies have been developed (New strategies in controlling drug resistance:
• Bone marrow transplantation: Imatinib and Dasatinib are tremendously effective at blocking disease progression; however, they are generally not thought to be curative. Currently, the only known curative therapy is bone marrow transplant. However, this is only a potential option for younger patients in good performance status.
• Stopping Imatinib after cytogenetic remission: in patients who achieve a complete molecular response (CMR), Bcr-Abl transcripts are no longer detectable by PCR.
• Allogeneic HCT (AlloHCT): use for those who are intolerant to TKIs or have mutations such as the T315I mutation, which can produce significant resistance to all of the clinically available TKIs. In addition, it seems that in high-risk or advanced-phase patients, a more aggressive approach would be to combine second-generation TKIs initially, followed by AlloHCT. [25] .
• Autologous stem cell transplantation (auto-SCT): could also eliminate a Ph+ clone bearing a BCR-ABL kinase domain mutation, thus could be a promising therapeutic option for imatinib resistance.
• ATP non-competitive ABL TKI: useful for patients with the T315I mutation. Some examples are ON012380, the aurora kinase inhibitor MK-0457, and the p38 MAP kinase inhibitor BIRB796 [26] .
Current situation
Now a days, several estudies are being developed. For further information about the pipeline, check clinical trials.gov
Authors
Almudena Chaves Pérez; Julio César Corral Serrano; Cristina Murga Montesinos.
Some morphs and scenes were reproduced from the pages from David Canner. We acknowledge the help of Elisa Lucas.
References
- ↑ Yuan ZM, Huang Y, Ishiko T, Kharbanda S, Weichselbaum R, Kufe D. Regulation of DNA damage-induced apoptosis by the c-Abl tyrosine kinase. Proc Natl Acad Sci U S A. 1997 Feb 18;94(4):1437-40. PMID:9037071
- ↑ Wang JY. Regulation of cell death by the Abl tyrosine kinase. Oncogene. 2000 Nov 20;19(49):5643-50. PMID:11114745 doi:10.1038/sj.onc.1203878
- ↑ Nagar B, Bornmann WG, Pellicena P, Schindler T, Veach DR, Miller WT, Clarkson B, Kuriyan J. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571). Cancer Res. 2002 Aug 1;62(15):4236-43. PMID:12154025
- ↑ O'Hare T, Walters DK, Stoffregen EP, Sherbenou DW, Heinrich MC, Deininger MW, Druker BJ. Combined Abl inhibitor therapy for minimizing drug resistance in chronic myeloid leukemia: Src/Abl inhibitors are compatible with imatinib. Clin Cancer Res. 2005 Oct 1;11(19 Pt 1):6987-93. PMID:16203792 doi:10.1158/1078-0432.CCR-05-0622
- ↑ Ramakrishnan C, Dani VS, Ramasarma T. A conformational analysis of Walker motif A [GXXXXGKT (S)] in nucleotide-binding and other proteins. Protein Eng. 2002 Oct;15(10):783-98. PMID:12468712
- ↑ Johnson DA, Akamine P, Radzio-Andzelm E, Madhusudan M, Taylor SS. Dynamics of cAMP-dependent protein kinase. Chem Rev. 2001 Aug;101(8):2243-70. PMID:11749372
- ↑ Gajiwala KS, Wu JC, Christensen J, Deshmukh GD, Diehl W, Dinitto JP, English JM, Greig MJ, He YA, Jacques SL, Lunney EA, McTigue M, Molina D, Quenzer T, Wells PA, Yu X, Zhang Y, Zou A, Emmett MR, Marshall AG, Zhang HM, Demetri GD. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci U S A. 2009 Feb 3;106(5):1542-7. Epub 2009 Jan 21. PMID:19164557
- ↑ Leukemia research 34 (10): 1255–1268. doi:10.1016/j.leukres.2010.04.016. PMID 2053738
- ↑ Zhao X, Ghaffari S, Lodish H, Malashkevich VN, Kim PS. Structure of the Bcr-Abl oncoprotein oligomerization domain. Nat Struct Biol. 2002 Feb;9(2):117-20. PMID:11780146 doi:10.1038/nsb747
- ↑ http://atlasgeneticsoncology.org/Genes/ABL.html
- ↑ Liu Y, Gray NS. Rational design of inhibitors that bind to inactive kinase conformations. Nat Chem Biol. 2006 Jul;2(7):358-64. PMID:16783341 doi:10.1038/nchembio799
- ↑ Scheinfeld N. A comprehensive review of imatinib mesylate (Gleevec) for dermatological diseases. J Drugs Dermatol. 2006 Feb;5(2):117-22. PMID:16485879
- ↑ Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, Huntly B, Fabbro D, Fendrich G, Hall-Meyers E, Kung AL, Mestan J, Daley GQ, Callahan L, Catley L, Cavazza C, Azam M, Neuberg D, Wright RD, Gilliland DG, Griffin JD. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005 Feb;7(2):129-41. PMID:15710326 doi:S1535-6108(05)00028-0
- ↑ Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, Bochinski K, Hochhaus A, Griffin JD, Hoelzer D, Albitar M, Dugan M, Cortes J, Alland L, Ottmann OG. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006 Jun 15;354(24):2542-51. PMID:16775235 doi:10.1056/NEJMoa055104
- ↑ Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004 Jul 16;305(5682):399-401. PMID:15256671 doi:10.1126/science.1099480
- ↑ Cortes JE, Jones D, O'Brien S, Jabbour E, Ravandi F, Koller C, Borthakur G, Walker B, Zhao W, Shan J, Kantarjian H. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010 Jan 20;28(3):398-404. doi: 10.1200/JCO.2009.25.4920. Epub 2009, Dec 14. PMID:20008620 doi:10.1200/JCO.2009.25.4920
- ↑ Khoury HJ, Cortes JE, Kantarjian HM, Gambacorti-Passerini C, Baccarani M, Kim DW, Zaritskey A, Countouriotis A, Besson N, Leip E, Kelly V, Brummendorf TH. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood. 2012 Apr 12;119(15):3403-12. doi: 10.1182/blood-2011-11-390120. Epub 2012 , Feb 27. PMID:22371878 doi:10.1182/blood-2011-11-390120
- ↑ http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm318203.htm
- ↑ Manley PW, Cowan-Jacob SW, Mestan J. Advances in the structural biology, design and clinical development of Bcr-Abl kinase inhibitors for the treatment of chronic myeloid leukaemia. Biochim Biophys Acta. 2005 Dec 30;1754(1-2):3-13. Epub 2005 Sep 8. PMID:16172030 doi:10.1016/j.bbapap.2005.07.040
- ↑ O'Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, Adrian LT, Zhou T, Huang WS, Xu Q, Metcalf CA 3rd, Tyner JW, Loriaux MM, Corbin AS, Wardwell S, Ning Y, Keats JA, Wang Y, Sundaramoorthi R, Thomas M, Zhou D, Snodgrass J, Commodore L, Sawyer TK, Dalgarno DC, Deininger MW, Druker BJ, Clackson T. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009 Nov 6;16(5):401-12. PMID:19878872 doi:10.1016/j.ccr.2009.09.028
- ↑ Frame D. New strategies in controlling drug resistance in chronic myeloid leukemia. Am J Health Syst Pharm. 2007 Dec 15;64(24 Suppl 15):S16-21. PMID:18056927 doi:10.2146/ajhp070483
- ↑ An X, Tiwari AK, Sun Y, Ding PR, Ashby CR Jr, Chen ZS. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review. Leuk Res. 2010 Oct;34(10):1255-68. PMID:20537386 doi:10.1016/j.leukres.2010.04.016
- ↑ Olivieri A, Manzione L. Dasatinib: a new step in molecular target therapy. Ann Oncol. 2007 Jun;18 Suppl 6:vi42-6. PMID:17591830 doi:10.1093/annonc/mdm223
- ↑ Boschelli, F.; Arndt, K.; Gambacorti-Passerini, C. (2010). "Bosutinib: a review of preclinical studies in chronic myelogenous leukaemia". European journal of cancer (Oxford, England : 1990) 46 (10): 1781–1789.doi:10.1016/j.ejca.2010.02.032. PMID 20399641. edit)
- ↑ Cortes J, Kim DW, Raffoux E, Martinelli G, Ritchie E, Roy L, Coutre S, Corm S, Hamerschlak N, Tang JL, Hochhaus A, Khoury HJ, Brummendorf TH, Michallet M, Rege-Cambrin G, Gambacorti-Passerini C, Radich JP, Ernst T, Zhu C, Van Tornout JM, Talpaz M. Efficacy and safety of dasatinib in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blast phase. Leukemia. 2008 Dec;22(12):2176-83. doi: 10.1038/leu.2008.221. Epub 2008 Aug 28. PMID:18754032 doi:10.1038/leu.2008.221
- ↑ An X, Tiwari AK, Sun Y, Ding PR, Ashby CR Jr, Chen ZS. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review. Leuk Res. 2010 Oct;34(10):1255-68. PMID:20537386 doi:10.1016/j.leukres.2010.04.016