Sandbox 215
From Proteopedia
| |||||||||
| 2obd, resolution 2.10Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , , , , , | ||||||||
| Gene: | CETP (Homo sapiens) | ||||||||
| |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Cholesteryl ester transfer protein (CETP), which is also called plasma lipid transfer protein belongs to a family of proteins that allow lipid transfer. The human cholesteryl ester transfer protein is a hydrophobic glycoprotein which is mainly synthesized in the liver, but also in the intestine, spleen and adrenal glands. The gene coding for this protein is located on the sixteen chromosome.
In the plasma, CETP plays an important role in the transport of cholesteryl esters from the atheroprotective high-density lipoproteins (HDL) to the atherogenic lower-density lipoproteins (LDL) and also mediates the transport of triglycerides from LDL to HDL. Most of the time, CETP facilites homoexchange by exchanging a triglyceride for another triglyceride and a cholesteryl ester for a cholesteryl ester between lipoproteins. However, CETP can also promote heteroexchange.
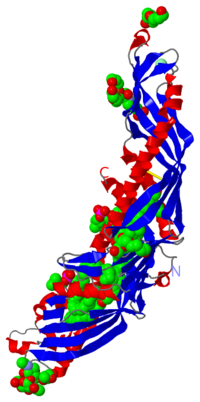
The cristal structure of CETP, in complex with four bound lipid molecules at 2,2 Å resolution shows a long tunnel traversing the core of the molecule. This tunnel has two large openings allowing lipids access and each opening is plugged by an amphiphilic phosphatidylcholine.
Contents |
Structure
| |||||||||||
Mechanism allowing neutral-lipid and phospholipid transfer [1] [2]
In the plasma, CETP often binds high density lipoproteins (HDL) and engages the tranfer of neutral lipids, such as cholesteryl ester and triglyceride among lipoprotein particles. The concave structure of CETP is the only surface able to bind a lipoprotein. Other surfaces of CETP are not able to bind them. It indicates that CETP can only bind one lipoprotein at a time. It means that CETP operates as carrier: CETP accepts neutral lipids from a donor particule and releases them to an acceptor particule.
Binding to a HDL particle, which is cholesteryl ester rich allows CETP to fill with cholesteryl esters, because one or two cholesteryl esters can enter the tunnel and an equal amount of triglyceride is deposited into HDL. Then the tunnel is refilled with two phospholipids (one at each end) that permit the protein to dissociate from HDL and to return to the acqueous phase. CETP also adopts a structural change by twisting its barrel around the central β-sheet in order to bind VLDL particules which are larger than HDL particules. Binding to a VLDL particle, which is triglyceride rich permits the release of the bound phospholipid. That allows one or two triglycerides to enter the tunnel and an equal amount of cholesteryl ester can be deposit into VLDL. The triglyceride-bound dissociates from VLDL. It carries two phospholipids from the surface of VLDL and travels through the acqueous plasma in order to rebind a HDL particle and to permit the release of the bound phospholipid. Then the cycle can continue.
CETP inhibition [2]
LDL particles are constitued of a single apolipoprotein which is apo-B100. They are often called “bad cholesterol” because a high rate of LDL leads to a deposit of cholesterol as plaques in arteries and that can cause cardiovascular problems.
Unlike to LDL, HDL particles are considered as “good cholesterol” because they are able to remove cholesterol, via the plasma, from peripheral tissues to the liver, where it will be degraded. They are constitued of apolipoproteins A-I and apo A-II. In fact, a high level of HDL can prevent from the accumulation of cholesterol in the plasma and avoid the developpement of cardiovascular diseases and atherosclerosis. That's why a promising solution to increase the level of HDL is the inhition of CETP.
Natural inhibitors
In the human plasma some natural inhibitors of CETP can be found: like Apo-CI, which his main role is to inhibit CETP, probably by altering the elecric charge of HDL. [3]
Pharmaceutical inhibitors
The pharmaceutical industry tries to develop inhibitors of CETP in order to decrease the risk of cardivascular diseases. The goal of these inhibitors is to increase the concentration of HDL and decrease the concentration of LDL by blocking cholesteryl esters and triglyceride tranfer. Several inhibitors were found: the first is Torcetrapib followed of Anacetrapib, Dalcetrapib and Evacetrapib.
Torcetrapib was developed by Pfizer in order to treat hypercholesterolemia (high cholesterol levels) and prevent atherosclerosis. Torcetrapib managed to reduce CETP activity and succeeds in increasing the level of HDL. However, in stage-III clinical trials, Torcetrapib causes significant changes in vital signs: like increases the blood pressure, the concentration of sodium, bicarbonate and aldosterone. The explanations for this unexpected result remain unclear but perhaps increased binding of torcetrapib-CETP complexes to HDL may interfer with the anti-cardiovascular diseases (anti-CVD) activity of HDL causing the death of many persons. That's why, in 2006, this inhibitor was halted.
Unlike to Torcetrapib, the other which still are in clinical trial do not have any side effects. Evacetrapib is developed by Eli Lilly & Company and seems to be the more promising inhibitor of CETP. Evacetrapib is able to achieve a complete inhibition of CETP, resulting in a significant elevation of HDL and reduction of LDL without increases blood pressure and changes aldosterone concentration. Evacetrapib is actually in phase II clinical trials. [4]
External ressources
References
- ↑ 1.0 1.1 1.2 1.3 Qiu X, Mistry A, Ammirati MJ, Chrunyk BA, Clark RW, Cong Y, Culp JS, Danley DE, Freeman TB, Geoghegan KF, Griffor MC, Hawrylik SJ, Hayward CM, Hensley P, Hoth LR, Karam GA, Lira ME, Lloyd DB, McGrath KM, Stutzman-Engwall KJ, Subashi AK, Subashi TA, Thompson JF, Wang IK, Zhao H, Seddon AP. Crystal Structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nature Structural & Molecular Biology. 2007 Feb;14(2):106-13. Epub 2007 Jan 21. PMID: 17237796 doi:10.1038/nsmb1197
- ↑ 2.0 2.1 James A Hamilton & Richard J Deckelbaum. Crystal structure of CETP: new hopes for raising HDL to decrease risk of cardiovascular disease? Nature Structural & Molecular Biology 14, 95 - 97 (2007). PMID: 17277799 doi:10.1038/nsmb0207-95
- ↑ Philip J. Barter, H. Bryan Brewer, Jr, M. John Chapman, Charles H. Hennekens, Daniel J. Rader and Alan R. Tall. Cholesteryl Ester Transfer Protein : A Novel Target for Raising HDL and Inhibiting Atherosclerosis. Arterioscler Thromb Vasc Biol 2003, 23:160-167: originally published online January 2, 2003.doi: 10.1161/01.ATV.0000054658.91146.64.
- ↑ Cao G, Beyer TP, Zhang Y, Schmidt RJ, Chen YQ, Cockerham SL, Zimmerman KM, Karathanasis SK, Cannady EA, Fields T, Mantlo NB. Evacetrapib is a novel, potent, and selective inhibitor of cholesteryl ester transfer protein that elevates HDL cholesterol without inducing aldosterone or increasing blood pressure. The Journal of Lipid Research, December 2011. PMID: 21957197. doi: 10.1194/jlr.M018069
Proteopedia Page Contributors and Editors
BLEU Mélusine, GOEPFERT Laetitia