Sandbox Reserved 1477
From Proteopedia
| This Sandbox is Reserved from November 5 2018 through January 1, 2019 for use in the course "CHEM 4923: Senior Project taught by Christina R. Bourne at the University of Oklahoma, Norman, USA. This reservation includes Sandbox Reserved 1471 through Sandbox Reserved 1478. |
To get started:
More help: Help:Editing |
This page is reserved for Bo
Contents |
Introduction
The topic is the study of the enzyme glucose oxidase (GOX) from aspergillus niger. It is not an enzyme from human body. Instead, it is found in the cells of fungus. These types of enzymes catalysts the oxidation of β-D-glucose to δ-gluconolactone and hydrogen peroxide (which is happening in human body) [1].
GOX has a “considerable commercial importance” in biosensors according to the essay. This type of enzyme is wildly used as biosensor. Since it could react with glucose with a great selectivity and produce hydrogen peroxide, the amount of hydrogen peroxide is going to be a function of the initial amount of the glucose ideally. For is reason, it is used for measuring the amount of concentration of glucose quantitatively. One of the application could be measuring the concentration of blood glucose [2]. GOX could be (actually had already been) widely used in quick blood glucose tests, which is essential to patients with diabetes. However, the usage of this enzyme is not exactly limited to the medical area. A good method of reflecting the concentration of glucose could also be used in food industry in measuring and controlling the sugar amount in food manufacturing since most types of natural-sourced carbohydrates in this case, such as sucrose and lactose, contain at least one molecule of glucose [3].
Two PDB files mentioned in the essay, 1cf3 and 1gpe, which are two GOX's from different organisms, aspergillus niger' and Penicillium Amagasakiense. They have exactly the same types of ligands. The most important ligand is FAD cofactor, which assists the oxidation of β-D-glucose. This essay was published in 1999, which is very old in the scale of science.
Structure
|
The structure of this PDB file (1cf3) contains only one chain with 4 different types of ligands. This is a 583-residue-long enzyme. The structure is tested by X-ray diffraction.
Another GOX from Penicillium Amagasakiense also tested with X-ray diffraction method in the same essay has two identical chains in the PDB file which is the regular form of this type of enzyme. They have 81% sequence similarity and also very similar structures (which could be seen from Fig. 1).
Each chain of the enzyme contains a (the red part). The molecules are tightly but non-covalently bonded to the enzyme [4]. The residues of the active sites, Tyr-73, Phe-418, Trp-430, Arg-516, Asn-518, His-520 and His-563, locate around the cofactor. The cofactors locate at the interface between two chains of the enzyme (which could be seen from Fig. 2), covered by an “irregular two-stranded antiparallel-sheet structure formed by residues 75-98”, which prevents the cofactors from being released [5]. Generally speaking, the FAD molecules are tightly “covered” or “surrounded” by the enzyme. The binding sites with FAD of both enzymes (GOX’s from Aspergillus niger and Penicillium Amagasakiense) are almost identical with several exceptions of hydrogen-bonds-forming residues such as His-78 and Thr-110. The positions of the two residues mentioned above are replaced by Gln (78) and Ser (100) in the GOX from Penicillium Amagasakiense. Flavin O4’s of the FAD cofactors in both enzymes are connected to the 110th residue (either Thr or Ser) and the Gly-108 residue [6].
12 hydrogen bonds formed with the glucose (9 with residues, 2 with water molecules and 1 with the cofactor) as well as the hydrophobic effect of residues Phe-418 and Trp-430 stabilize the active site of the GOX [7]. As mentioned, the critical hydrogen bonds are the three hydrogen bonds formed between Arg-516 and 3-OH of the glucose based on the discussion in the function part.
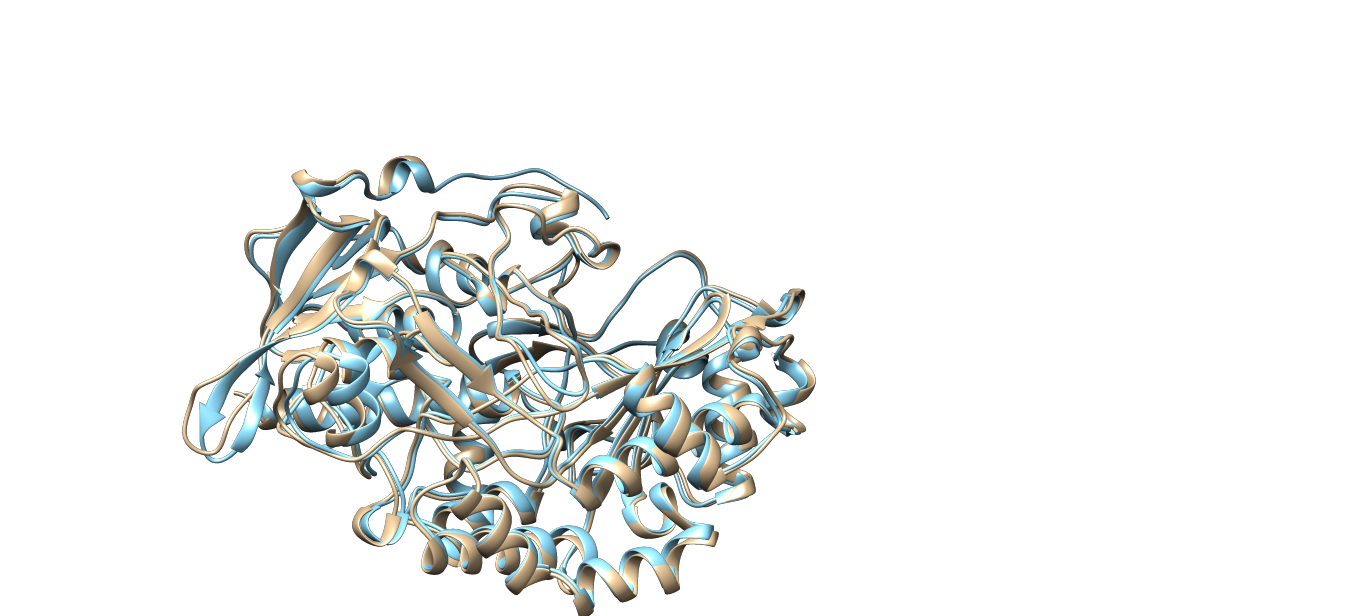 Fig. 1 Comparison between the structures of glucose oxidase from aspergillus niger (PDB code:1cf3, the tan part) and penicillium amagasakiense (PDB code: 1gpe, the cyan part). One of the chains of PDB file 1gpe was hidden.
Fig. 1 Comparison between the structures of glucose oxidase from aspergillus niger (PDB code:1cf3, the tan part) and penicillium amagasakiense (PDB code: 1gpe, the cyan part). One of the chains of PDB file 1gpe was hidden.
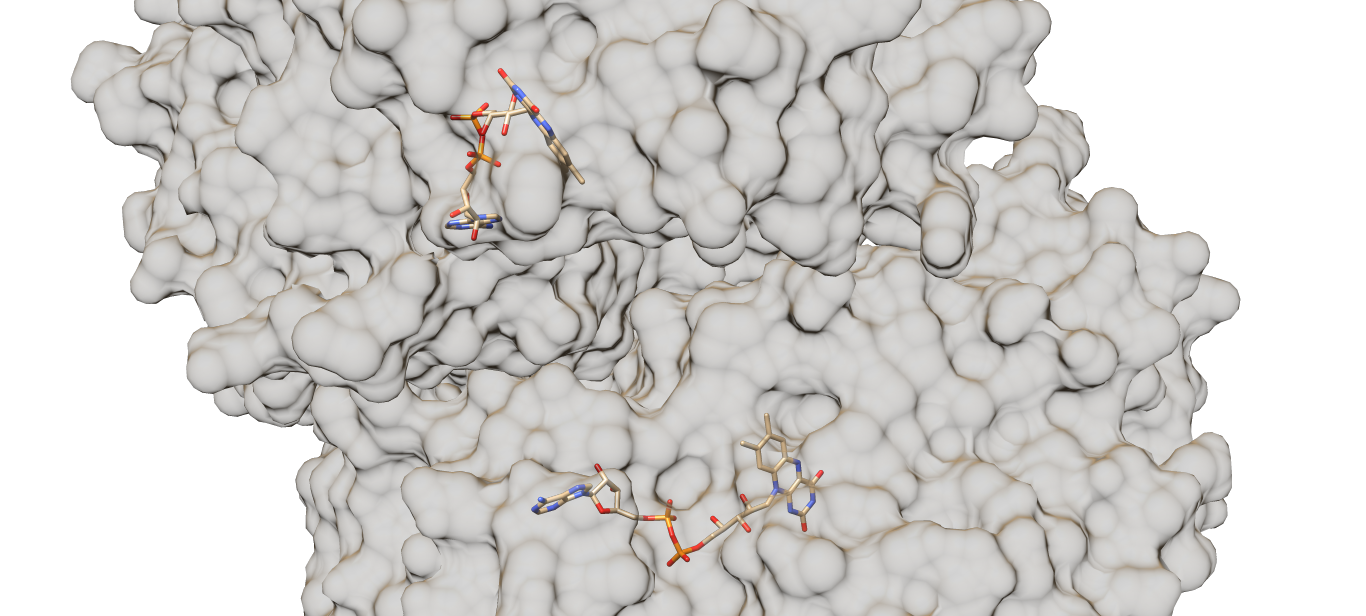 Fig. 2 Relative position of two FAD molecules of the penicillium amagasakiense (PDB code: 1gpe) with transparent surface, which will hopefully give a rough image about how the two chains of the enzyme are arranged. Since the 1cf3 file (GOX from aspergillus niger) only have one chain given, the 1gpe (GOX from penicillium amagasakiense) was used instead.
Fig. 2 Relative position of two FAD molecules of the penicillium amagasakiense (PDB code: 1gpe) with transparent surface, which will hopefully give a rough image about how the two chains of the enzyme are arranged. Since the 1cf3 file (GOX from aspergillus niger) only have one chain given, the 1gpe (GOX from penicillium amagasakiense) was used instead.
Media:Movie compressed compressed.mp4 The super compressed version of my movie. Sorry I have to compress it a lot in order to fit the size of the file allowed to upload.
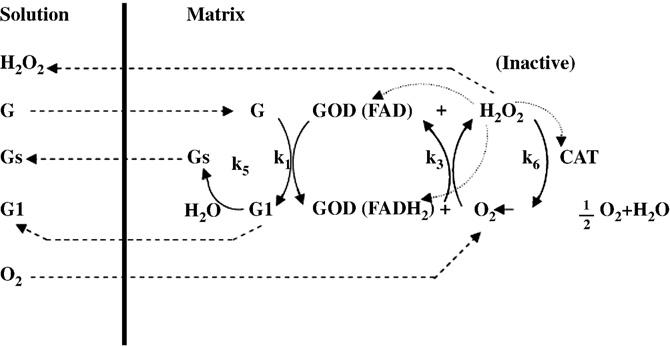
Function
GOX in fungus functions as an anti-bacterial and anti-fungal reagent by producing the hydrogen peroxide. The production of hydrogen peroxide produced allows the fungus competing with other types of bacteria or fungi, especially those which could not produce hydrogen peroxide. The fungus with GOX will be protected by the catalase in its cell, which could break down hydrogen peroxide “peacefully” without hurting the cell. The existence of hydrogen peroxide in the concentration of micromolar level with the presence of GOX could inhibit the growth of cells [8]. This fact makes the fungus with GOX very competitive comparing fungi without GOX.
Another product, δ-gluconolactone, could be hydrolyzed either enzymatically or non-enzymatically to gluconic acid [9]. This reaction is going to decrease the pH value of the environment of the fungus, which, according to Dr. Wong, makes GOX able to function as a preservative.
The enzyme is very selective. Specific constant, which has a lot of different names in different essays, is used to describe the rate of the reaction. It is defined as kcat/Km , which is a much fairer and more representative way comparing to mention k_cat or Km only because the reaction rate is expressed as v=(kcat[E]tS)/(Km+[S]). Comparing to the specific constant of the enzyme binding to the β-D-glucose, When binding to the 2-deoxyglucose, which has a similar structure as the original reactant β-D-glucose, the enzyme shows a 10-fold lower specific constant; when binding to the D-mannose, which also has a great structural similarity comparing with β-D-glucose, the enzyme shows a 400-fold lower specific constant; when binding to D-Galactose, the enzyme shows a 1000-fold lower specific constant; when binding to D-Xylose, the enzyme shows a 3000-fold lower specific constant [10].
Energetic
FAD acts as an electron carrier during the reaction. The GOX-FAD form of the enzyme is reduced to the GOX-FADH2 form during the reaction. The FAD oxidizes the β-D-glucose to δ-gluconolactone and being reduced to FADH2; the oxygen molecule is reduced to the hydrogen peroxide with the electrons transferred. This process is supported by a protonated His residue, which thus functions best at lower pH. Although the mechanism of the reaction had been clearly studied, the actual roles of the residues of the active sites are still unclear [11]. Fig. 3 below is a overall view of the reaction cited from Dr. Bankar's work.
Fig. 3 A picture cited from Glucose oxidase — An overview by Sandip B. Bankar. It gives an overall view of the reaction.
References
- ↑ Wohlfahrt G, Witt S, Hendle J, Schomburg D, Kalisz HM, Hecht HJ. 1.8 and 1.9 A resolution structures of the Penicillium amagasakiense and Aspergillus niger glucose oxidases as a basis for modelling substrate complexes. Acta Crystallogr D Biol Crystallogr. 1999 May;55(Pt 5):969-77. PMID:10216293
- ↑ Tian K, Prestgard M, Tiwari A. A review of recent advances in nonenzymatic glucose sensors. Mater Sci Eng C Mater Biol Appl. 2014 Aug 1;41:100-18. doi:, 10.1016/j.msec.2014.04.013. Epub 2014 Apr 13. PMID:24907743 doi:http://dx.doi.org/10.1016/j.msec.2014.04.013
- ↑ Bankar SB, Bule MV, Singhal RS, Ananthanarayan L. Glucose oxidase--an overview. Biotechnol Adv. 2009 Jul-Aug;27(4):489-501. doi:, 10.1016/j.biotechadv.2009.04.003. Epub 2009 Apr 15. PMID:19374943 doi:http://dx.doi.org/10.1016/j.biotechadv.2009.04.003
- ↑ Bankar SB, Bule MV, Singhal RS, Ananthanarayan L. Glucose oxidase--an overview. Biotechnol Adv. 2009 Jul-Aug;27(4):489-501. doi:, 10.1016/j.biotechadv.2009.04.003. Epub 2009 Apr 15. PMID:19374943 doi:http://dx.doi.org/10.1016/j.biotechadv.2009.04.003
- ↑ Wohlfahrt G, Witt S, Hendle J, Schomburg D, Kalisz HM, Hecht HJ. 1.8 and 1.9 A resolution structures of the Penicillium amagasakiense and Aspergillus niger glucose oxidases as a basis for modelling substrate complexes. Acta Crystallogr D Biol Crystallogr. 1999 May;55(Pt 5):969-77. PMID:10216293
- ↑ Wohlfahrt G, Witt S, Hendle J, Schomburg D, Kalisz HM, Hecht HJ. 1.8 and 1.9 A resolution structures of the Penicillium amagasakiense and Aspergillus niger glucose oxidases as a basis for modelling substrate complexes. Acta Crystallogr D Biol Crystallogr. 1999 May;55(Pt 5):969-77. PMID:10216293
- ↑ Witt S, Wohlfahrt G, Schomburg D, Hecht HJ, Kalisz HM. Conserved arginine-516 of Penicillium amagasakiense glucose oxidase is essential for the efficient binding of beta-D-glucose. Biochem J. 2000 Apr 15;347(Pt 2):553-9. PMID:10749686
- ↑ Wong CM, Wong KH, Chen XD. Glucose oxidase: natural occurrence, function, properties and industrial applications. Appl Microbiol Biotechnol. 2008 Apr;78(6):927-38. doi: 10.1007/s00253-008-1407-4., Epub 2008 Mar 11. PMID:18330562 doi:http://dx.doi.org/10.1007/s00253-008-1407-4
- ↑ Bankar SB, Bule MV, Singhal RS, Ananthanarayan L. Glucose oxidase--an overview. Biotechnol Adv. 2009 Jul-Aug;27(4):489-501. doi:, 10.1016/j.biotechadv.2009.04.003. Epub 2009 Apr 15. PMID:19374943 doi:http://dx.doi.org/10.1016/j.biotechadv.2009.04.003
- ↑ Wohlfahrt G, Witt S, Hendle J, Schomburg D, Kalisz HM, Hecht HJ. 1.8 and 1.9 A resolution structures of the Penicillium amagasakiense and Aspergillus niger glucose oxidases as a basis for modelling substrate complexes. Acta Crystallogr D Biol Crystallogr. 1999 May;55(Pt 5):969-77. PMID:10216293
- ↑ Witt S, Wohlfahrt G, Schomburg D, Hecht HJ, Kalisz HM. Conserved arginine-516 of Penicillium amagasakiense glucose oxidase is essential for the efficient binding of beta-D-glucose. Biochem J. 2000 Apr 15;347(Pt 2):553-9. PMID:10749686