Sandbox Reserved 1649
From Proteopedia
|
Contents |
NR2A (2A5S)
NR2A is a protein which is a part of NMDA receptors, that are heterodimer channels composed of four subunits. Indeed, NMDA receptors are made of the association between two NR2 and two NR1 proteins. NMDA receptors play a key role in mammalian central nervous system, as they act in Ca2+ influx in synapses in response to glutamate and glycine binding. Their role is essential for learning and memory. Variety of NR2 allows modulation of NMDAr. In the other hand, NMDA receptors are related to AMPA receptors in the same synapse.
Structure and Function
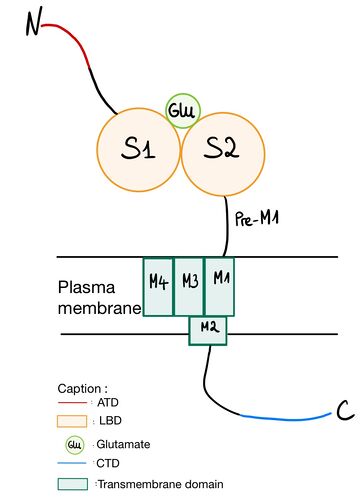
NR2A (GluN2A) is composed of an amino-terminal domain (ATD), segments S1 and S2 which formed ligand binding domain of glutamate, three transmembrane helices(M1, M3,and M4), a cytoplasmic re-entrant pore loop (M2), and an intracellular C-terminal domain (CTD).Amino-terminal domain (ATD)
The ATD is constituted by the first 383 amino acids of NR2A. ATD is an alpha and beta protein class. Its structure is bilobed and forms a clam-shell like structure which consists in two lobes linked by a flexible hinge region defining a central groove. [1] Zn2+ may insert between 2 lobes and induces closure of channel by changing conformation of ATD. Zn increases affinity of glutamate on the LBD which reminds the desensitization of AMPA and Kainate receptor. [2] [3] ATD allows to modulate NMDA receptor. The difference between various NR2 is mainly regulated by ATD. Indeed, the diversity of ATD can modulate traffic in endoplasmic reticulum and then affect the localization of NMDAr. ATD of NR2A increases glutamate affinity, controls channel’s opening with high probability and opens duration, controls glutamate deactivation time course.[4]
Ligand binding domain (LBD)
LBD is constituted of two domains S1 (located juste upstream M1 transmembrane domain) and S2 and has affinity for or sometimes Glycine. Positive charge of amino-group of the agonist binds to negative charges residue of the pocket D731. In GlurR, negative charge amino acid is a E731 and is able to form salt bridge with agonist. In NR2A D731 (which corresponds to ) is not able to do salt bridge with amino group because aspartate is one methylene lacking to do it. Amino group of agonist is stabilized by water mediated hydrogen bonds to amino acid Y761 (which corresponds to ) and E413 (which correspond to ).. The high affinity for glutamate agonist may be because of Van der Walls contact between γ-carboxylate group of glutamate and Y730 of S2 domain which is conserved in NR2 protein.[5] Amino-group of glutamate also interacts with and .
On the other hand, from this domain interact with NR1 (see NR1/NR2A complex part).
Transmembrane domain
The transmembrane domain is organized into 4 parts (from M1 to M4). M1 connects the N-terminal domain to M2. M2 forms a reentrant loop contributing to the pore. The S1 segment of the N-terminal domain intertwines with the S2 segment of the GlnBP-type domain in the extracellular loop M3 - M4 to form the glutamate binding pocket. On the other hand, desensitization of NMDA receptors is affected by residues near or inside the binding pocket as well as by residues in M2 that line the pore and the M3 loop - M4 is not responsible for the specificity of the NR2 subunit of glycine independent desensitization. [6] M2 loop is a channel-lining loop and located in transmembrane domain. Two asparagines are located on N site of the domain and block Mg2+ and are permeable of Ca2+ [7] Structurally, there is a small loop of 150 amino acids between M3 and M4. Ethanol acts as an inhibitor on NMDAr. phenylalanine at position 639 in the M3 part of the transmembrane domain of NR2A interacts with the latter.[8]
CTD
CTD domain is the less conserved of the NR2 domains. Like ATD, it allows different localization of NMDAr thanks to reticulum endoplasmic trafficking. It is indispensable for receptor surface dynamic and activation of specific signaling. CTD phosphorylation can modulate NMDAr, for instance it is useful for endocytosis during glutamate binding on LBD. [7]
Regulation
A high quantity of stress can lead to the overactivation of NMDA receptors. Therefore, a regulation can occur on NMDA receptors in order to avoid any neuronal injury. [9] This very important regulation can act at the level of NR2A subunit.
For now, scientists know that regulation can occur in several ways: either a modification directly affects the functioning of the receptor, or the quantity of receptors at the surface of the neuron by modifying NR2A itself or not.
• Inhibition by Zn2+
Zn2+ exercises a control on the functioning of NR2A. Zn2+ is considered to be an endogenous inhibitor of NMDA receptors. Indeed, the NTD of NR2A subunit forms a binding site for Zn2+.
The binding of Zn2+ on NR2A generates an allosteric modulation of NMDA receptor, leading to its closure. The molecular mechanisms by which the NTD can communicate the inhibitory change of conformation to the rest of the receptor is still unknown. Nevertheless, the LBD was identified as a major intermediate between the NTD to the channel gate. It seems that H+ would also inhibits NR2A by conformational changes. Then, it has been proposed that zinc exerts its inhibitory action on NR1/NR2A receptors through an enhancement of tonic proton inhibition. According to this model, the presence of protons is required for zinc inhibition to occur. [9]
• Inhibition by CK2 indirectly on NR2A
Casein Kinase 2 (CK2) determines the NR2 subunit content of synaptic NMDA. Indeed, CK2 phosphorylates NR2B subunit in response to activity, what regulates the number of synaptic NR2A and NR2B. Indeed, CK2 phosphorylation leads to NR2B endocytosis and remove NR2B from synapses, resulting in an increase in synaptic NR2A expression. [10]
• Inhibition by palmitoylation directly on NR2A
NR2A has two distinct consensus cysteine clusters in its C-terminal region, which are susceptible to be palmitoylated.
If the first cluster is palmitoylated, the activity of some tyrosine kinases is increased. Their phosphorylations lead to enhanced stability of NMDA receptors on the cell surface, but also ensure their proper surface delivery from the Golgi apparatus. In contrast, depalmitoylation of this cluster decreases the surface NMDA receptors, because there are less stably associated with the plasma membrane.
On the contrary, the palmitoylation of the second cluster by distinct palmitoyl transferases causes receptors accumulation in the Golgi apparatus and so reduces receptor surface expression.
Since palmitoylation is reversible, by modulating phosphorylation on NR2A thanks to this dual palmitoylation, the traffiking of NMDA receptors can be regulated. Palmitoylation of Cystein cluster I regulates NMDA receptor internalization whereas palmitoylation of Cys cluster II retains the receptor in the Golgi apparatus. It is important because it controls the number of effective NMDA receptors at the surface of the cell, which has an impact on the neuronal function. [11]
NR1/NR2A complex [[1]]
NR2A can be found in some NMDA receptors, in which NR2A is associated to NR1. NMDA receptors are necessarily heteromers of 4 subunits, organized as a dimer of dimers. These dimers are heterodimers constituted with a glycine binding NR1 and a glutamate binding subunits NR2.
NR1 and NR2A are assembled in a dimer ([Complex NR1/NR2a]), arranged in a back-to-back fashion, thanks to interactions between three different domains on each subunit: sites I, II and III.
- Site II: The link between NR2A and NR1 is made by at least three amino acids: E530 () makes a salt bridge with R755 of NR1, F524 () binds K531 of NR1 by a hydrogen bond on the backbone carbonyl oxygen, and P257.
- Sites I and III: the binding is established by hydrophobic residues (I514 (), V526 (), L777, L780 present on helices D and J), or by polar contacts
Depending on the kind of NR2 (A-D) linked to NR1, the affinity of NR1 for glycine can be affected. Moreover, for a particular combination of NR1 and NR2 subunits, a negative cooperativity has been observed between glycine and glutamate binding. This leads to consider a possible allosteric coupling between NR1 and NR2. Thus, the importance of the structure of NR2A to make contacts with NR1 is obvious. But mechanistic explanation about the role that subunit-subunit contacts might have in NMDA receptor activity has not been found yet. [4]
Since the opening of the NMDA channel requires both glutamate and glycine, respectively detected by NR2 and NR1, the association between NR1 and NR2A is primordial.
Mutations
The C-terminal truncation of NR2 subunits in NMDA receptors is an interesting mutation. Indeed, the C-terminal truncation of NR2A influences the functioning of the NMDA receptor. Several experiments were carried out and made it possible to conclude on this influence. First, mice with truncated NR2A subunits have been shown to still have a functional receptor channel, but NR2A ΔC / ΔC mice appear to be altered in the cellular signal transduction events involved in the induction of LTP (potentiation long-term). Despite the presence of the full-length NR2B subunit, the C-terminal truncation of the NR2A subunit altered the signal transduction mediated by NMDAR. Second, the C-terminal truncation of NR2A has been shown to alter fear in a particular setting. Indeed, the experiment was to put mutant and wild mice through stepwise avoidance training and put them in water. Thus, the researchers concluded that mutant mice have a significantly reduced latency time to leave the safe platform compared to wild mice and that mutant mice exhibit water balance deficits.
The C-terminal truncation of NR2A therefore has consequences on synaptic plasticity and synaptic reorganization during the recording of hippocampal LTP, on the conditioning of fear and also on motor coordination. [12]
NMDA receptors are inhibited by ethanol. Mutagenesis of an F residue in the transmembrane domain has made it possible to characterize the role of the latter in this inhibition. On the other hand, the phenylalanine residue at position 639 is replaced by an alanine in the transmembrane domain (in part M3) and the results show significantly less inhibition by ethanol of NMDA compared to wild type receptors.[8]
Disease
Anti-nuclear antibodies produced in systemic or systemic lupus erythematosus (SLE, SLE) interact with the NR2A subunit of the NMDA receptor. This is because the Asp / Glu-Trp-Asp / Glu-Tyr-Ser / Gly pentapeptide is a molecular mimic of double-stranded DNA, so antibodies produced in a patient with SLE recognize this pentapeptide. The latter is also present in the structure of NR2A. Thus, these antibodies cross-interact with NR2A and therefore the NMDA receptor. This interaction can signal neuronal death by an excitotoxic mechanism. More generally, NMDA receptor dysfunction is implicated in multiple brain disorders, such as stroke, chronic pain, and schizophrenia. [13]
References
- ↑ Zhu S, Stroebel D, Yao CA, Taly A, Paoletti P. Allosteric signaling and dynamics of the clamshell-like NMDA receptor GluN1 N-terminal domain. Nat Struct Mol Biol. 2013 Apr;20(4):477-85. doi: 10.1038/nsmb.2522. Epub 2013 Mar, 3. PMID:23454977 doi:http://dx.doi.org/10.1038/nsmb.2522
- ↑ Paoletti P, Perin-Dureau F, Fayyazuddin A, Le Goff A, Callebaut I, Neyton J. Molecular organization of a zinc binding n-terminal modulatory domain in a NMDA receptor subunit. Neuron. 2000 Dec;28(3):911-25. doi: 10.1016/s0896-6273(00)00163-x. PMID:11163276 doi:http://dx.doi.org/10.1016/s0896-6273(00)00163-x
- ↑ Gielen M. [Molecular operation of ionotropic glutamate receptors: proteins that mediate the excitatory synaptic neurotransmission]. Med Sci (Paris). 2010 Jan;26(1):65-72. doi: 10.1051/medsci/201026165. PMID:20132777 doi:http://dx.doi.org/10.1051/medsci/201026165
- ↑ 4.0 4.1 Yuan H, Hansen KB, Vance KM, Ogden KK, Traynelis SF. Control of NMDA receptor function by the NR2 subunit amino-terminal domain. J Neurosci. 2009 Sep 30;29(39):12045-58. doi: 10.1523/JNEUROSCI.1365-09.2009. PMID:19793963 doi:http://dx.doi.org/10.1523/JNEUROSCI.1365-09.2009
- ↑ Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005 Nov 10;438(7065):185-92. PMID:16281028 doi:10.1038/nature04089
- ↑ Krupp JJ, Vissel B, Heinemann SF, Westbrook GL. N-terminal domains in the NR2 subunit control desensitization of NMDA receptors. Neuron. 1998 Feb;20(2):317-27. doi: 10.1016/s0896-6273(00)80459-6. PMID:9491992 doi:http://dx.doi.org/10.1016/s0896-6273(00)80459-6
- ↑ 7.0 7.1 Franchini L, Carrano N, Di Luca M, Gardoni F. Synaptic GluN2A-Containing NMDA Receptors: From Physiology to Pathological Synaptic Plasticity. Int J Mol Sci. 2020 Feb 24;21(4). pii: ijms21041538. doi: 10.3390/ijms21041538. PMID:32102377 doi:http://dx.doi.org/10.3390/ijms21041538
- ↑ 8.0 8.1 Ronald KM, Mirshahi T, Woodward JJ. Ethanol inhibition of N-methyl-D-aspartate receptors is reduced by site-directed mutagenesis of a transmembrane domain phenylalanine residue. J Biol Chem. 2001 Nov 30;276(48):44729-35. doi: 10.1074/jbc.M102800200. Epub 2001, Sep 25. PMID:11572853 doi:http://dx.doi.org/10.1074/jbc.M102800200
- ↑ 9.0 9.1 Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron. 2010 Sep 23;67(6):984-96. doi: 10.1016/j.neuron.2010.08.011. PMID:20869595 doi:http://dx.doi.org/10.1016/j.neuron.2010.08.011
- ↑ doi: https://dx.doi.org/110.1016/j.neuron.2010.08.011
- ↑ Hayashi T, Thomas GM, Huganir RL. Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron. 2009 Oct 29;64(2):213-26. doi: 10.1016/j.neuron.2009.08.017. PMID:19874789 doi:http://dx.doi.org/10.1016/j.neuron.2009.08.017
- ↑ Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, Hvalby O, Jensen V, Paulsen O, Andersen P, Kim JJ, Thompson RF, Sun W, Webster LC, Grant SG, Eilers J, Konnerth A, Li J, McNamara JO, Seeburg PH. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell. 1998 Jan 23;92(2):279-89. doi: 10.1016/s0092-8674(00)80921-6. PMID:9458051 doi:http://dx.doi.org/10.1016/s0092-8674(00)80921-6
- ↑ DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001 Nov;7(11):1189-93. doi: 10.1038/nm1101-1189. PMID:11689882 doi:http://dx.doi.org/10.1038/nm1101-1189