Sandbox Reserved 1781
From Proteopedia
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Your Heading Here (maybe something like 'Structure')
This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs. You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction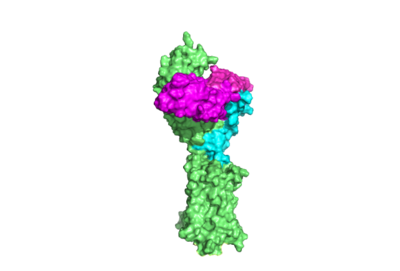 Figure 1. TSHR with TSH bound. The extracellular and transmembrane domains of the GPCR are shown in green, the hinge region in cyan, and thyrotropin bound in magenta. PDB:7UTZ Thyroid hormones exercise essential functions related to activity of thyroid cells as well as metabolic processes and oxygen consumption[3]. Misregulation of thyroid hormone levels is the cause of many disorders related to hypo- or hyperthyroidism. Thus, understanding the signaling of synthesis and release of these hormones will have applications in treating thyroid hormone disorders[3]. The initiation of the synthesis and release of these hormones is caused by the glycoprotein, thyroid stimulating hormone (TSH), which is released by the anterior pituitary gland[3]. The release of TSH from the anterior pituitary is regulated by thyroid-releasing hormone (TRH) which is released by the hypothalamus. When stimulated by TRH, the anterior pituitary releases TSH. When stimulated by TSH, the thyroid gland will produce and release the the thyroid hormones T4 and T3. T3 is the "active form" of the hormone, however it accounts for only 20% of the thyroid hormone that is released after stimulus by TSH. The T4 that predominates in release from the thyroid will be converted to T3 in the bloodstream. High levels of T3 and T4 can negatively regulate the release of TSH from the anterior pituitary, constituting a negative feedback loop [4]. The thyrotropin receptor (TSHR)is a G-protein coupled receptor on the surface the thyroid gland cells. TSHR is responsible for binding TSH and transduces signal to initiate synthesis and release of thyroid hormones. In addition to TSH, autoantibodies may also bind to TSHR causing inhibition or activation of its desired function. (Figure 1)[5][6] StructureOverviewThe thyrotropin receptor has an extracellular domain (ECD) that is composed of a leucine rich repeat domain (LRRD) as well as a hinge region. This hinge region links the ECD to the seven transmembrane helices (7TM domain), which span from the extracellular domain to the intracellular domain [7]. Thyrotropin binding causes a conformational change in the ECD that is transduced through the transmembrane helices. In the active state, the ECD is in the "up" position, while in the inactive state, the ECD is in the "down" state, closer to the cell membrane. A "push-pull" mechanism is proposed to be responsible for the ECD's conformational change between active and inactive states. In the "push" model, TSH binds to the receptor and sterically clashes with the cellular membrane forcing the ECD up away from the membrane. In the pull model, a short α-helix interacts with TSH to pull the ECD up. The active (up) form of the ECD causes a conformation shift in the TMD which causes differential interactions with a heterotrimeric G-protein, initiating intracellular signaling[5]. Hinge Region and P10 PeptideWithing TSHR, the hinge region is a scaffold for the attachment of the LRRD to the 7TMD. The hinge region also impacts TSH binding potency and intracellular cyclic adenosine monophosphate (cAMP) levels, mediated by the activation of the GPCR[8]. The hinge region's hinge helix with the p10 peptide through disulfides. The p10 peptide is a conserved sequence that spans from the last beta sheet of the LRRD to the first transmembrane helix (TM1) and is an intramolecular agonist for conformational shifts in the 7TMD helices[9]. The disulfides between LRRD, hinge helix, and p10 are critical to TSH signaling as they transduce signal from the ECD through the hinge helix to the p10 peptide. Upward movement of the LRRD, caused by TSH binding, will cause rotation of the hinge helix and subsequent movement of the p10 peptide leading to movement of the transmembrane helices which will cause activation of the G-protein. In addition to activation, the hinge region plays an important role in tightly binding TSH. Residues 382-390 of the hinge region adopt a short helix containing two key residues. Y385 from TSHR is buried into a hydrophobic pocket of TSH while D386 forms a salt bridge with R386 of the hormone. Interactions that assist in the stable binding of TSH to the TSHR allow more potent activation of the receptor[5]. Even with these key functions, the hinge region itself is not absolutely required for receptor activation[9]. The hinge region functions as a point of attachment to the 7TMD for the LRRD, and its ability to rotate allows for LRRD shifts between up (active state) and down (inactive state) positions. The hinge region also contains key residues that stabilize TSH binding. Disulfides that the hinge helix makes with the LRRD an p10 act as an important communication medium between the extracellular environment and an intramolecular agonist which directly effects conformational shifts in the 7TMD. 7 Transmembrane HelicesThe ECD of TSHR is anchored to the membrane through seven transmembrane helices (7TMD), characteristic of GPCRs. Conformational changes in the 7TMD activate intracellular G-protein signaling[7]. Once TSH binds, changes to the p10 peptide are transmitted to the 7TMD. Specifically, hinge helix rotation causes displacement of the p10 peptide that allows the seventh transmembrane helix (TM7) to migrate towards the center of the 7TMD to increase van Der Waals contacts. Additionally, K660 of TM7 forms a stabilizing ionic interaction with E409 of the p10 region. Hinge helix movement also rearranges Y279 relative to I486 on the neighboring extracellular loop 1 (ECL1) helix, which links two transmembrane helices and is located extracellularly. Substitution of these residues leads to substantial shifts in the activation of the thyrotropin receptor. Structurally guided mutagenic studies have shown that replacing isoleucine with a more sizeable phenylalanine decreases TSH signaling potency[9][10].The sixth transmembrane helix of TSHR moves outward from the center of the 7TMD to facilitate α-helix 5 of the α-subunit of the G protein (Gα)[9][11]. Gα is activated for intracellular signaling when GDP is exchanged for GTP and dissociates from the γ- and β-subunits of the G-protein (Gγ and Gβ) to bind with other target proteins. Activation of the Gα is caused by conformational shifts in the 7TMD and three intracellular loops which directly interact with the G-protein[7]. Conformational shifts in transmembrane helices are the mechanism of changing interactions of the G-protein with the receptor. Activation of the G-protein is caused by these conformational shifts. RelevanceStructural highlightsThis is a sample scene created with SAT to color by Group, and another to make a transparent representation of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
|
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 3.2 Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.
- ↑ Pirahanchi Y, Toro F, Jialal I. Physiology, Thyroid Stimulating Hormone. [Updated 2022 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499850/
- ↑ 5.0 5.1 5.2 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287-384. doi: 10.1016/s0083-6729(08)60658-5. PMID: 7709602.
- ↑ 7.0 7.1 7.2 Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086
- ↑ Yumiko Mizutori, Chun-Rong Chen, Sandra M. McLachlan, Basil Rapoport, The Thyrotropin Receptor Hinge Region Is Not Simply a Scaffold for the Leucine-Rich Domain but Contributes to Ligand Binding and Signal Transduction, Molecular Endocrinology, Volume 22, Issue 5, 1 May 2008, Pages 1171–1182, https://doi.org/10.1210/me.2007-0407
- ↑ 9.0 9.1 9.2 9.3 Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-
- ↑ Virginie Vlaeminck-Guillem, Su-Chin Ho, Patrice Rodien, Gilbert Vassart, Sabine Costagliola, Activation of the cAMP Pathway by the TSH Receptor Involves Switching of the Ectodomain from a Tethered Inverse Agonist to an Agonist, Molecular Endocrinology, Volume 16, Issue 4, 1 April 2002, Pages 736–746, https://doi.org/10.1210/mend.16.4.0816
- ↑ Goricanec, D., Stehle, R., Egloff, P., Grigoriu, S., Plückthun, A., Wagner, G., & Hagn, F. (2016). Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proceedings of the National Academy of Sciences, 113(26). https://doi.org/10.1073/pnas.1604125113

