Sandbox Reserved 303
From Proteopedia
| This Sandbox is Reserved from January 10, 2010, through April 10, 2011 for use in BCMB 307-Proteins course taught by Andrea Gorrell at the University of Northern British Columbia, Prince George, BC, Canada. |
To get started:
More help: Help:Editing |
Thermolysin
| |||||||||
| 1y3g, resolution 2.10Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , | ||||||||
| Non-Standard Residues: | |||||||||
| Activity: | Thermolysin, with EC number 3.4.24.27 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
is a protein that is isolated from the thermophile Bacillus thermoproteolyticus. [1] This protein is characterized by a remarkable stability under heat and protein denaturants. [2]
Structure
Thermolysin comes from the superfamily of metalloproteases and was one of the first metalloproteases to have its structure solved crystallographically.[3]
Thermolysin consists of one polypeptide strand of 316 residues.[4] There are 12 and 17 . The helices consist of 41% or the protein, with the srands making up 18%.[4] Thermolysin is a bilobal structure and the active site is located at a pronounced cleft formed at the junction of the protein's two domains.[1]
The structure does not contain any stabilizing disulfide bonds.[5] Instead, Thermolysin binds four that are necessary for its thermal stability. These four ions increase the intrinsic thermostability of the protein and protect its surface loops against autolysis.[1]
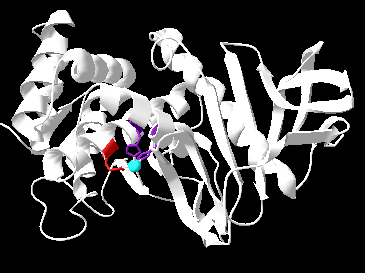
One is also present in the protein. This zinc ion is located at the active site of Thermolysin.[3]
Figure 1: Depicts the active site of thermolysin with zinc (in blue) connected to HIS 142, HIS 146 (in purple), and GLU 166 (in red).
Function
Thermolysin functions to catalyze both the cleavage and the synthesis of peptide bonds.[1]
The zinc molecule present at the active site is essential for the enzyme's function. It exists in an approximate tetrahedral coordination, bound to three amino acids (HIS 142, HIS 146, GLU 166) and water. During its activity, an incoming substrate will displace this water molecule towards GLU 143. [1] GLU 143 is thought to help activate this same water molecule, which will attack the substrate.[3]
The zinc ion activates the scissile amide bond towards attack by water and stabilizes the resulting tetrahedral intermediate.[3] The metal is able to react with a number of functional groups that act as inhibitors. Some of these groups include thiols, ketones, hydroxamic acids, anionic groups such as carboxylates ans phosphinates, and silanediols.[3] The design of metalloprotease inhibitors such as for thermolysin provides a deeper understanding of enzymatic activity and ligand-receptor interaction.[3]
Works Cited
- ↑ 1.0 1.1 1.2 1.3 1.4 Matthews, B. (1988). "Structural Basis of the Action of Thermolysin and Related Zinc Peptidases." Acc. Chem. Res. 1988, 21, 333-340.
- ↑ Vita, C., Dalzoppo, D. & Fontana, A. (1985). "Limited Proteolysis of Thermolysin by Subtilisin: Isolation and Characterization of a Partially Active Enzyme Derivative." Biochemistry, 24, 1798-1806.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Juers DH, Kim J, Matthews BW, Sieburth SM. Structural analysis of silanediols as transition-state-analogue inhibitors of the benchmark metalloprotease thermolysin. Biochemistry. 2005 Dec 20;44(50):16524-8. PMID:16342943 doi:10.1021/bi051346v
- ↑ 4.0 4.1 PDB 1Y3G. http://www.rcsb.org/pdb/explore/remediatedSequence.do?structureId=1Y3G
- ↑ Fontana, A., Vita, C., Boccu', E., & Veronese, FM. (1977). "A Fluorimetric Study of the Role of Calcium Ions in the Stability of Thermolysin." Biochem. J. 165, 539-545.