Sandbox Reserved 382
From Proteopedia
| This Sandbox is Reserved from September 14, 2021, through May 31, 2022, for use in the class Introduction to Biochemistry taught by User:John Means at the University of Rio Grande, Rio Grande, OH, USA. This reservation includes 5 reserved sandboxes (Sandbox Reserved 1590 through Sandbox Reserved 1594). |
To get started:
More help: Help:Editing. For an example of a student Proteopedia page, please see Photosystem II, Tetanospasmin, or Guanine riboswitch. |
Contents |
Aromatase
|
Introduction
Aromatase belongs to the cytochrome P450 family (CYP). During aromatization reactions, aromatase forms an electron-transfer complex with its partner, NADPH-cytochrome P450 reductase. This enzyme is localized in the endoplasmic reticulum of the cell, and tissue specific promoters regulate its activity.[1] In a number of species, including humans, aromatase can be found throughout the body in places such as the brain, gonads, blood vessels, endometrium, skin, bone and tissues including the placenta and adipose tissue.[2]
Function
The primary function of aromatase is to produce estrogens by aromatizing androgens. Aromatase is the only known enzyme in vertebrates capable of catalyzing the aromatization of a six-membered ring[3]. Aromatase converts androstenedione to estrogen and testosterone to estradiol.[4] Aromatase is also a key enzyme in the biosynthesis of estrogens through a process called steroidogenesis. This enzyme helps produce the female sex hormone, estrogen, that helps to fuel the growth of hormone receptor-positive breast cancer. There are many environmental factors that affect the activity of the aromatase enzyme and disrupt its function. Factors that increase the activity of the enzyme include age, obesity, gonadotropins, insulin, alcohol and smoking.[4]
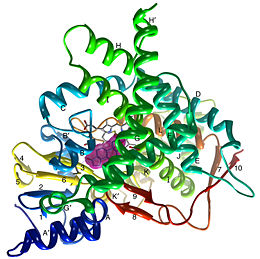
Structure
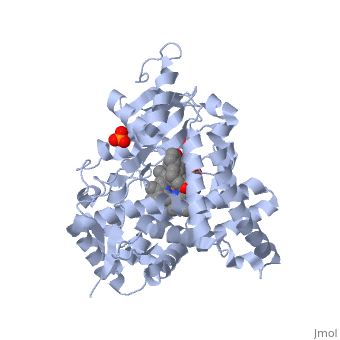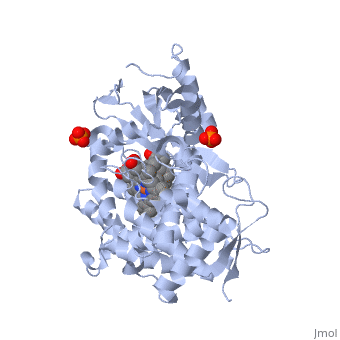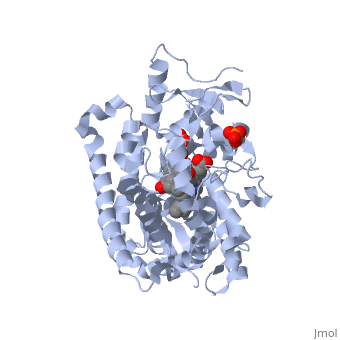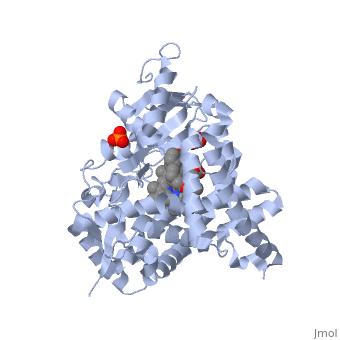
Due to the membrane-bound nature of mammalian CYP enzymes, the structural characterization is extremely difficult. Aromatase is a monomeric enzyme composed of a heme-prosthetic group and a single polypeptide chain consisting of 503 amino acid residues.[3] One important feature of CYPs is the iron-containing group at the enzyme active site.[5] The is within the porphyrin and is considered the reaction center of the enzyme. The docks in a region adjacent to the porphyrin. Aromatase is anchored to the endoplasmic reticulum by the amino terminal transmembrane domain. The tertiary structure of aromatase includes twelve major α-helices and ten β-strands.[3] An androstenedione molecule is bound in the active site of the enzyme. The active site of the enzyme can be found in the distal cavity of the heme-binding pocket. A ribbon diagram displaying the overall structure of the human placental aromatase is shown to the left. The amino terminus starts at residue 45 and is shown in dark blue. The carboxyl terminus ending at residue 496 is shown in red. The helices are labeled A-L and the sheets are labeled 1-10 accordingly. The heme group and the bound ligand are shown in the center of the protein. [3]
Aromatase Inhibitors
Inhibitors of aromatase stop estrogen production in post-menopausal women. This action is done by blocking the aromatase enzyme by turning the hormone, androgen, into small amounts of estrogen.[6] There are three aromatase inhibitors that are often used in the treatment of breast cancer:
- Arimidex (Anastrozole)
- Aromasin (Exemestane)
- Femara (Letrozole)
Aromatase inhibitors are unable to stop ovaries from producing estrogen; therefore, these inhibitors only work in post-menopausal women.
Disorders
- Aromatase Enzyme Deficiency
Aromatase deficiency is rare in humans; however, if aromatase is nonfunctional due to a mutation, estrogen synthesis cannot occur. Affected females are diagnosed at birth because of the obvious characteristics of pseudohermaphroditism. During the childhood of these girls, delayed bone maturation can occur along with cystic ovaries. However, affected males are diagnosed later in life because there are not obvious birth defects. Clincal symptoms such as a tall physique, delayed bone maturation, epiphyseal closure, bone pain, and excess adiposy.[7]
- Aromatase Excess Syndrome
Research shows a rare disorder caused by excessive aromatase activity that can cause familial gynecomastia and feminization of both sexes. This can be inherited in an autosomal dominant manner, affecting females and males differently. Females with this disorder showed signs of isosexual precocity and/or macromastia. Males showed characteristics of heterosexual precocity and/or gynecomastia.[8]
References
- ↑ Kagawa N. Efficient expression of human aromatase (CYP19) in E. coli. Methods Mol Biol. 2011;705:109-22. PMID:21125383 doi:10.1007/978-1-61737-967-3_7
- ↑ Conley A, Hinshelwood M. Mammalian aromatases. Reproduction. 2001 May;121(5):685-95. PMID:11427156
- ↑ 3.0 3.1 3.2 3.3 3.4 Ghosh, D., Griswold, J., Erman, M., Pangborn, W. " X-ray Structure of Human Aromatase Reveals An Androgen-Specific Active Site" Journal of Steroid Biochemistry and Molecular Biology. [Online] 2010,Vol. 118, Issue 4-5, p197-202. [1]
- ↑ 4.0 4.1 "Aromatase Products" [2]
- ↑ Favia AD, Cavalli A, Masetti M, Carotti A, Recanatini M. Three-dimensional model of the human aromatase enzyme and density functional parameterization of the iron-containing protoporphyrin IX for a molecular dynamics study of heme-cysteinato cytochromes. Proteins. 2006 Mar 1;62(4):1074-87. PMID:16395678 doi:10.1002/prot.20829
- ↑ "Aromatase Inhibitors" [3]
- ↑ Jones ME, Boon WC, McInnes K, Maffei L, Carani C, Simpson ER. Recognizing rare disorders: aromatase deficiency. Nat Clin Pract Endocrinol Metab. 2007 May;3(5):414-21. PMID:17452968 doi:10.1038/ncpendmet0477
- ↑ Stratakis CA, Vottero A, Brodie A, Kirschner LS, DeAtkine D, Lu Q, Yue W, Mitsiades CS, Flor AW, Chrousos GP. The aromatase excess syndrome is associated with feminization of both sexes and autosomal dominant transmission of aberrant P450 aromatase gene transcription. J Clin Endocrinol Metab. 1998 Apr;83(4):1348-57. PMID:9543166