Sandbox Reserved 385
From Proteopedia
| This Sandbox is Reserved from September 14, 2021, through May 31, 2022, for use in the class Introduction to Biochemistry taught by User:John Means at the University of Rio Grande, Rio Grande, OH, USA. This reservation includes 5 reserved sandboxes (Sandbox Reserved 1590 through Sandbox Reserved 1594). |
To get started:
More help: Help:Editing. For an example of a student Proteopedia page, please see Photosystem II, Tetanospasmin, or Guanine riboswitch. |
|
Tamoxifen and Breast Cancer
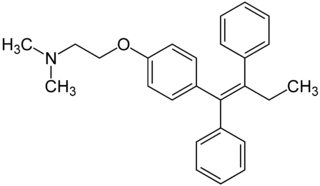
Recent studies have shown that men and women with high estrogen levels, coupled with an already high risk of developing breast cancer, are at a higher risk of the disease occurring.[1] Estrogen is necessary in many areas of the body. It gives cells permission to grow, including cancer cells. Estrogen is regulated through an activated estrogen receptor transcription factor. These transcription factors in the higher risk patients can activate oncogenes that accelerate cancer cell growth. A recent hypothesis suggests that a new way to prevent and treat breast cancer is to change the way estrogen binds to the receptor. The drug Tamoxifen acts as a competitive inhibitor of estrogen and the estrogen receptor. Those with a higher risk of breast cancer who undergo treatment with Tamoxifen show low breast tissue density, which suggests a lower breast cancer risk. Tamoxifen is a smaller molecule that mimics the shape of estrogen, allowing it to bind tightly to the estrogen receptor.
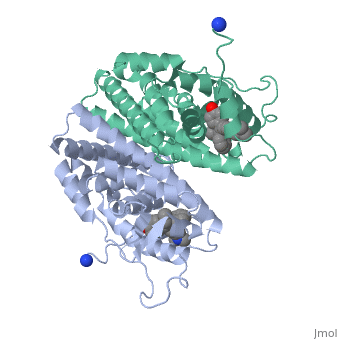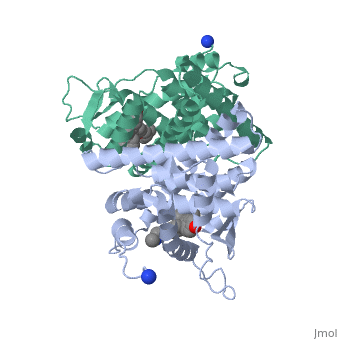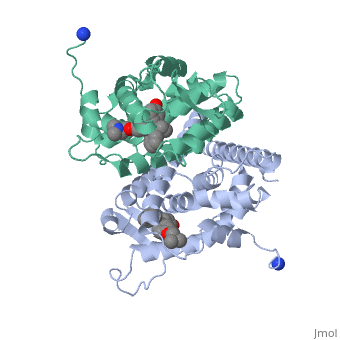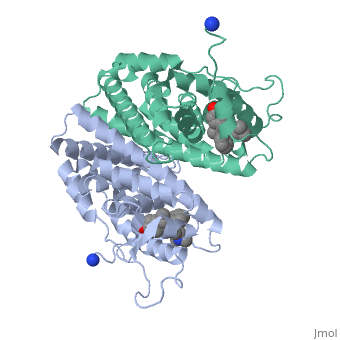
Tamoxifen and the Estrogen Receptor
Tamoxifen lacks the second OH group as well as a tail containing oxygen and nitrogen on the ring.[1] Tamoxifen binds to the ligand binging domain of the estrogen receptor, which leads to a conformational shift.[1] The conformational change causes the to shift into an adjacent coactivator.[2] This site is essential for estrogen to do its job. Without the coactivator binding, the receptor remains inactive. Conformational changes also occur due to the new hydrophobic interactions between helices 3 and 11. These newly formed hydrophobic interactions lead to a cascade effect of conformational changes across the molecule. The new side chain also causes conformational changes since one of the rings in Tamoxifen is shoved deeper into the pocket. Tamoxifen also provides one less hydrogen bond in the pocket compared to estrogen, causing helices 3, 8, and 11 to extend. With the coactivator site blocked, there is a halt in proliferation, meaning that there is no cell growth.
Tamoxifen, the Drug
Tamoxifen is a precursor for the drug that binds to the estrogen receptor, making it a prodrug. The actual drug is 4-hydroxyltamoxifen, which has a greater affinity for the estrogen receptor than Tamoxifen alone. The FDA approved this prodrug 30 years ago to prevent breast cancer in high risk patients. Tamoxifen is classfied as a Seletive Estrogen Receptor Modulator (SERM), meaning that it selectively blocks or activates the activity of estrogen on specific cells, such as breast cancer cells. While Tamoxifen is used to block estrogen acivity in breast cells, it also activates estrogen activity in other cells, such as bone and liver cells.
- ↑ 1.0 1.1 1.2 Skafar, D., and S. Koide. "Understanding the Human Estrogen Receptor-alpha Using Targeted Mutagenesis." Molecular and Cellular Endocrinology 246.1-2 (2006): 83-90.
- ↑ Pecorak, Sara, and Tom Susman. "Tamoxifen, Diethylstilbesterol and the Estrogen Receptor Ligand Binding Region." (04). Web. <http://biology.kenyon.edu/BMB/Chime2/2001/estrogen/FRAMES/start.htm>.
- ↑ Wikipedia.org. http://en.wikipedia.org/wiki/Tamoxifen (accessed December 9th, 2011).