Sandbox Reserved 490
From Proteopedia
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Estrogen ReceptorIntroductionThe activity of estrogen and estrogen receptors has been linked to several cancers, including breast cancer, ovarian cancer, and colon cancer, as well as skeletal and cardiovascular diseases. The use of antagonist or agonist compounds with regards to the ligand-binding effect on the estrogen receptor has been used as a treatment method for breast cancer and osteoporosis. [1] Estrogen receptors (ER) belong to the super family of nuclear receptor proteins (NRP). Nuclear receptor proteins are a diverse group of proteins located in the nucleus. These proteins bind to specific ligands and undergo conformational changes, whereby they can then act as transcription factors to up-regulate or down-regulate certain genes. Nuclear hormone receptors (NHR) are a subgroup of NRPs that have hormone ligands. Estrogen receptors are nuclear hormone receptors whose ligand is estrogen.[2] NRPs are found only in animals. Mouse and rat models are commonly used to research the function of ER due to the similarity of ER function. [3] There are two types of estrogen receptors: estrogen receptor-α and estrogen receptor-β. There has been much research done on both ER-α and ER-β, and it is still unclear exactly how each functions. However, like all NRPs, the ER proteins have a highly conserved DNA-binding domain, a specific ligand-binding domain, and a highly variable N-terminus region. In ER proteins, the ligand-binding domain is located near the C-terminal region of the protein. Once bound, the mechanism of transcriptional regulation is dependent upon what co-factors and other signalling molecules are present in the cell.[4]
StructureEstrogen receptors have two important structural components: the DNA-binding domain and the ligand-binding domain. The N-terminus region of the protein is variable, and this region undergoes many conformational changes when the protein binds to the estrogen ligand and to other cofactors that determine the DNA binding complex.[5] DNA-Binding domain[6]
This is the structure of the estrogen receptor when its DNA binding domain is complexed to DNA. It binds as a to the appropriate DNA sequence. Each dimer consists of . The are located on the face of the . These residues follow a modified zinc finger motif. The Glu-25, Lys-28, Lys-32, and Arg-33 residue side chains interact with the base pairs of the DNA. Other polar side chains interact less specifically with the phosphate backbone of the DNA. The DNA sequence that the dimerized estrogen receptor binds to is a palindromic sequence. This reflects the symmetric dimerization of the protein. This structure was determined using X-Ray Crystallography.
The ligand-bound estrogen receptor is also a . Each unit has 12 . The faces of the helices participating in the dimerization process face each other. The ligand-binding domain of each unit involves from helices 7, 8, and 9 in the binding pocket. Most of the residues are hydrophobic, to interact with the hydrophobic portions of the estrogen steroid. The Glu-353 and His-524 hydrogen bond directly with the hydroxyl groups on the estrogen ligand (see image). The ligand used to determine this structure was estradiol, which is the physiologically appropriate ligand. Estradiol, or estrogen, has the following structure:
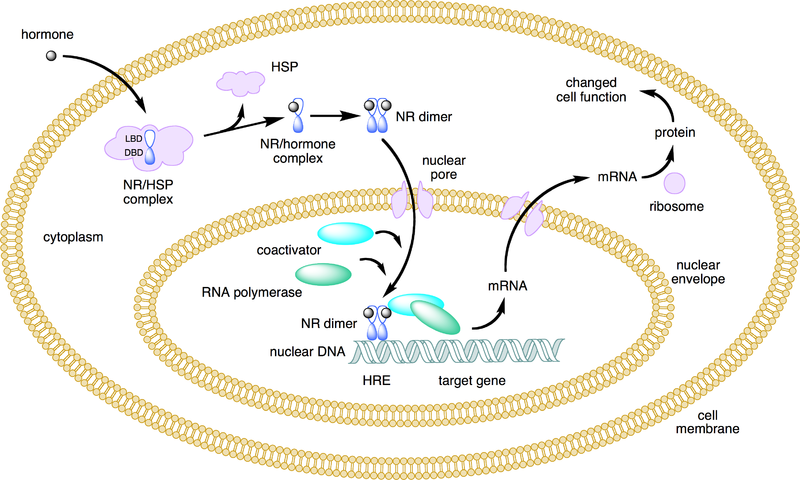
Mechanism of Action[8]Two general mechanisms of action have been proposed for ER proteins: Genomic and non-genomic. Much of research has focused on the genomic mechanism of action, which is still not clearly understood. Genomic Mechanism The genomic mechanism of action occurs within the nucleus of the cell and can be either ligand-dependent or independent. The ligand-dependent mechanism requires the estrogen ligand to diffuse into the cell and then into the nucleus, bind to the LBD of the ER, and induce a conformational change in the receptor protein. The receptor protein then recruits specific co-factors and molecules and assembles a DNA-binding complex, which binds to specific DNA promoter or repressor sequences, depending on the type of cell. Ligand-independent action of the ER occurs when some factor (for example, a growth factor) induces a kinase cascade that ultimately phosphorylates the ER and induces the protein to dimerize and act upon DNA and gene transcription. The ER may act as a transcription factor itself, or it may enhance the activity of other transcription factors. For example, ER has been shown to influence the activity of NFKb in transcribing interleukin-6. These genomic mechanisms are relatively slow.
This mechanism is still largely undefined. However, it has been suggest that outside of the nucleus, the occurrence of estrogen binding to ER may induce a rapid physiological change. However, it is unknown if this happens at the plasma membrane, withing the cytoplasm, or through the action of an unknown intermediary protein. The following image summarizes the ligand-dependent genomic mechanism of ER, which is the most well known mechanism: Clinical Applications[9]Estrogen and estrogen receptor proteins are known to have a wide variety of functions in multiple different tissue types. It is commonly known that estrogen acts as a morphogen, turning on genes that regulate sex differentiation and development. However, estrogen also plays an important role in other systems, such as the skeletal and cardiovascular systems. The specific genes that are turned on or off by estrogen in each cell type are influenced by what other compounds are in the cell. For example, the array of molecules in cardiovascular tissue is different from that of skeletal tissue, and thus the action of the estrogen-bound ER proteins are tissue-specific. The function of estrogen receptors has played a key role in the development of treatment strategies for many diseases, most notably for breast cancer. Drugs targeting ERs in cancerous breast tissue are designed as antagonists, suppressing ER activity in the cancerous tissue. Drugs targeting ER-α have been particularly effective at treating breast cancer. Other cancers that are strongly correlated with estrogen and ER activity are ovarian cancer, colon cancer, prostate cancer, and endometrial cancer. Drugs designed to act as agonists to ERs, increasing ER activity, have been used to treat osteoporosis. The symptoms of neurodegenerative diseases such as stroke, Parkinson's, and Alzheimer's, and cardiovascular diseases have been shown to be alleviated by estrogen and increased estrogen receptor activity. Further research on the mechanism of estrogen receptor action is necessary to improve upon treatment methods. Understanding the recruitment of specific co-factors in different tissues, the importance of the ERα:ERβ ratio, and how the combination of these things can change a compound's ER antagonist and agonist action will perhaps enable us to treat diseases more specifically and with a smaller incidence of negative side effects. No effective clinical treatments have been developed to treat neurodegenerative diseases associated with estrogen activity. Research is needed to develop treatment for these and other diseases related to estrogen and estrogen receptor activity. References
|