Sandbox Reserved 684
From Proteopedia
| This Sandbox is Reserved from 30/08/2012, through 01/02/2013 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 636 through Sandbox Reserved 685. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://proteopedia.org/w/Help:Getting_Started_in_Proteopedia
Ornithine TranscarbamoylaseIntroduction(OTC) is an enzyme that catalyzes the reaction between carbamoyl phosphate and ornithine to form citrulline and phosphate. In plants and microbes, OTC is involved in arginine biosynthesis, but in mammals it is located in the mitochondria and is part of the urea cycle.[1] OTC is often associated with Ornithine transcarbamoylase deficiency (OTCD). OTCD is a common urea cycle disorder, and it is a genetic disorder which results in a mutated and ineffective form of the enzyme OTC. OTC is a trimer composed of a monomer and two substrate-binding domains, and it contains an active site in the middle of the protein with three ligands. StructureOTC is a trimer. The monomer unit has a CP-binding domain and an amino acid-binding domain. Each of the two discrete substrate-binding domains (SBDs) have an α/β topology with a central β-pleated sheet embedded in flanking α-helices. The are located at the interface between the protein monomers.[2]The crystal structure of human ornithine transcarbamylase (OTCase) complexed with carbamoyl phosphate (CP) and L-norvaline (NOR) has been determined to 1.9-A resolution. There are significant differences in the interactions of CP with the protein, compared with the interactions of the CP moiety of the bisubstrate analogue N-(phosphonoacetyl)-L-ornithine (PALO). The carbonyl plane of CP rotates about 60 degrees compared with the equivalent plane in PALO complexed with OTCase. This positions the side chain of NOR optimally to interact with the carbonyl carbon of CP. The mixed-anhydride oxygen of CP, which is analogous to the methylene group in PALO, interacts with the guanidinium group of Arg-92; the primary carbamoyl nitrogen interacts with the main-chain carbonyl oxygens of Cys-303 and Leu-304, the side chain carbonyl oxygen of Gln-171, and the side chain of Arg-330. The residues that interact with NOR are similar to the residues that interact with the ornithine (ORN) moiety of PALO. The side chain of NOR is well defined and close to the side chain of Cys-303 with the side chains of Leu-163, Leu-200, Met-268, and Pro-305 forming a hydrophobic wall. C-delta of NOR is close to the carbonyl oxygen of Leu-304 (3.56 A), S-gamma atom of Cys-303 (4.19 A), and carbonyl carbon of CP (3.28 A). Even though the N-epsilon atom of ornithine is absent in this structure, the side chain of NOR is positioned to enable the N-epsilon of ornithine to donate a hydrogen to the S-gamma atom of Cys-303 along the reaction pathway. Binding of CP and NOR promotes domain closure to the same degree as PALO, and the active site structure of CP-NOR-enzyme complex is similar to that of the PALO-enzyme complex. The structures of the active sites in the complexes of aspartate transcarbamylase (ATCase) with various substrates or inhibitors are similar to this OTCase structure, consistent with their common evolutionary origin.[3]The binding of carbamyl-P, L-ornithine, and its competitive inhibitor L-norvaline by ornithine transcarbamylase has been measured by gel filtration at 25° under the conditions used for the kinetic experiments. Carbamyl-P forms a reversible binary complex with the enzyme from both liver and Streptococcus faecalis, whereas L-norvaline is bound tightly only in the presence of carbamyl-P. There is the equivalent for each of one site per monomer. Two dissociation constants, differing 10-fold, are required to describe the data for the binding of both carbamyl-P and norvaline by the S. faecalis enzyme, and three, even more widely separated, for the binding of carbamyl-P to the bovine enzyme. L-Ornithine, which is also poorly bound to the free enzyme, is, in contrast to norvaline, tightly bound in the presence of phosphate.[4]
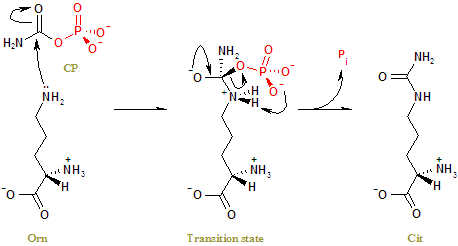
ImplicationsOrnithine transcarbamylase deficiency (OTCD) If a person is deficient in OTC, ammonia levels will build up, and this will cause neurological problems. Levels of the amino acids glutamate and alanine will be increased (as these are the amino acids that receive nitrogen from others). Levels of urea cycle intermediates may be decreased, as carbamoyl phosphate cannot replenish the cycle. The carbamoyl phosphate instead goes into the uridine monophosphate synthetic pathway. Here orotic acid (one step of this alternative pathway) levels in the blood are increased. A potential treatment for the high ammonia levels is to give sodium benzoate, which combines with glycine to produce hippurate, at the same time removing an ammonium group. Biotin also plays an important role in the functioning of the OTC enzyme and has been shown to reduce ammonia intoxication in animal experiment.[5] MechanismThe side chain amino group of Orn attacks the carbonyl carbon of CP nucleophillically, to form a tetrahedral transition state. A charge rearrangement then realeases Cit and Pi. N5-Phosphonoacetyl-l-ornithine (PALO, 1) is a bisubstrate transition-state analog which competitively inhibits ornithine transcarbamylase (OTC) in vitro.[6]Studies have also shown that N δ-(N′-sulfodiaminophosphinyl)-l-ornithine (PSOrn), with its three unique N-P bonds, represents a true transition state analogue for ornithine transcarbamoylases (OTC)[7]. Another inhibitor being studied is The inhibition of ornithine transcarbamoylase from Escherichia coli W by phaseolotoxin. In the presence of phaseolotoxin ornithine transcarbamoylase exhibited a transient phase of activity before a steady state.[8]
|