Sandbox Reserved 987
From Proteopedia
|
Contents |
Background
Cocaine use is a problem with approximately 21 million people abusing it worldwide. [1] Cocaine mostly affects the brain by inhibiting reuptake of norepinephrine, serotonin, and dopamine [2] With these neurotransmitters inhibited from reabsorbing it creates a sense of a "high" for the person using but the "crash" can lead to paranoia, restlessness, and anxiety. Cocaine Esterase (CocE) is the most efficient protein to hydrolyze the cocaine domain known to date in vivo.[3] Cocaine Esterase is used in bacterium Rhodococcus, which hydrolyzes the cocaine that it uptakes and uses it for carbons and nitrogens. Although this protein metabolizes cocaine in bacterium, it is sure to induce an immune response as it is foreign to the human body. This could mitigate the effects of CocE if a person had suffered from cocaine toxicity.
Structure
Cocaine Esterase is a globular protein that is expressed in the cytosol of Rhodococcus strain of bacteria, containing 574 residues divided into three domains. Domain I is a α/β hydrolase fold-containing domain consisting of residues 1-144 and residues 241-354 with the active site His-287. Domain II is α-helical, consisting of residues 145-240, making up seven helices inserted between β6 and β7 of Domain I. Helices two through six together form a five helix core with helices two and three combine to make a lid-like structure over the active site, His-287. Domain III is made up of residues 355-574 with mostly β-structure including a β-barrel-like core connected by 6 cross over loops, forming a jelly roll-like topology.[4]
is highlighted in blue
is highlighted in green
is highlighted in red
His 287 highlighted in blue
Mechanism
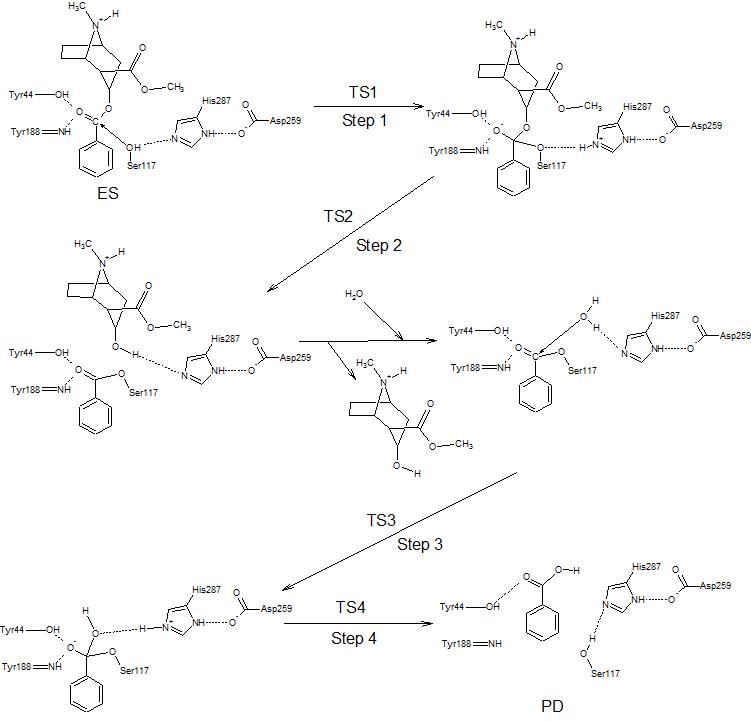
Cocaine esterase is used to catalyze the following reaction: cocaine + H2O ←→ ecgonine methyl ester + benzoate [5]
Cocaine esterase works in a four step mechanism.[6]
1. The lone pair on from Ser117 attacks the ester.
2. The extra pair of electrons on the oxygen reform the double bond and ecgonine methyl ester leaves.
3. A water molecules, stabilized by His287, attacks the ester again.
4. The extra pair of electrons reform the double bond again and this time break the bond to Ser117, regenerating the catalyst.
Mutations
The wild-type cocaine esterase is not stable at physiological temperature, which poses a problem for its use as a pharmaceutical drug. Mutations have been conducted in order to increase its thermostability. In vivo testing showed the wild-type having a 11 min half-life. A mutant having its threonine swapped for a arginine at position 172 (T172R) was found to last 9 times as long, and a mutant that further had its glycine swapped for a glutamine (T172R/G173Q) was found to last 24 times as long (~4h 20m)[7] Another mutant that has been explored is the L169K/G173Q mutant. This showed an in vivo half life of ~2h 20m.[8] L169K causes a large increase in half life up to 9h 30m, but reduced catalytic efficiency 4.5 fold.[9]
Medical Relevance
Cocaine is a tropane alkaloid produced by the South American plant Erythroxylon coca.[10] Cocaine’s psychological effects occur via binding to neurotransmitter reuptake transporters in the presynaptic nerve termini and blocking them, leading to prolonged presence of neurotransmitters in the synapse.[11] Large amounts of dopamine and serotonin in the synapse results in feelings of euphoria and wellbeing. This lingering increase in neurotransmitters within the synapse can lead to self-administration and, therefore, addiction. When cocaine is bound to noradrenergic transporters, elevated noradrenergic signaling occurs, resulting in increased heart rate (HR), blood pressure and vasoconstriction which are often seen in cocaine users.[12] Cocaine also binds cardiac and neuronal sodium channels leading to profound cardiovascular and central nervous system alterations that are frequently lethal.[13] Cocaine abuse is a serious public health problem. 50% of emergency room visits are due to illicit drug use, cocaine accounts for the majority of those cases.[14] Interestingly, despite cocaine’s broad use and addictive effects, there is no US FDA-approved medication for the treatment of cocaine abuse or toxicity. Physicians treat patients enduring cocaine toxicity with standard emergency room agents that control arrhythmias, convulsions and high blood pressure but not with drugs that directly address the toxic levels of cocaine present in the patient.
Finding a therapeutic agent to combat cocaine, such as small molecules has proven difficult. For this reason, the focus has been switched to cocaine esterases. For over a decade, cocaine esterases have been studied and numerous findings strongly suggest that bacterial cocaine esterases should provide a safe and effective method to rapidly eliminate the symptoms of acute cocaine intoxication in humans, as well as reducing addictiveness to the drug.
As an example, there was a test done involving rhesus-monkeys and the effects of CocE on the brain after cocaine uptake.[15] The study was the first to evaluate the effects of CocE on cocaine brain levels. Positron emission tomography (PET) neuroimaging was used to evaluate the time course of cocaine elimination from the brain of rhesus-monkeys in the presence and absence of CocE. PET scans of the rhesus-monkey brain show that just after 15 minutes of being exposed to CocE almost all the brain activity caused by cocaine has diminished; much more so opposed to saline solution. This data further supports the development of CocE for the treatment of acute cocaine toxicity.
References
- ↑ "200 Million People Use Illicit Drugs, Study Finds" Katie Moisse. abcNews Medical Unit. 6 January 2012
- ↑ "Cocaine Use and Its Effects" Joseph Goldberg. WebMD Substance Abuse and Addiction Health Center. 23 June 2013
- ↑ "Effects of cocaine esterase following its repeated administration with cocaine in mice" Mei-Chaun Ko, Diwahar Narasimhan, Aaron A. Berlin, Nicholas W. Lukacs, Roger K. Sunahara, James H. Woods. Drug and Alcohol Dependence 1 May 2009;101:202-09. doi: 10.1016/j.drugalcdep.2009.01.002
- ↑ Narasimhan, D.; Woods, J.H.; Sunahara, R.K. “Bacterial cocaine esterase: a protein-based therapy for cocaine overdose and addiction.” Future Med Chem. 2012, 4, 2: 137-150.
- ↑ Cocaine esterase From Wikipedia, the free encyclopedia
- ↑ Liu J., Zhao X., Yang W., Zhan G. C. (2011) Reaction mechanism for cocaine esterase-catalyzed hydrolysis of (+)- and (-)-cocaine: unexpected common rate-determining step. J. Phys. Chem. B. 115(17), 5017-5025.
- ↑ Gao D., Narasimhan D. L., Macdonald J., Brim R., Ko M., Landry D. W., Woods J. H., Sunahara R. K., Zhan C. (2009) Thermostable variants of cocaine esterase for long-time protection against cocaine toxicity. Mol. Pharmacol. 75, 318-323.
- ↑ Brim R. L., Nance M. R., Youngstrom D. W., Narasimhan D., Zhan C. G., Tesmer J. J. G., Sunahara, R. K., Woods J. W. (2010) A Thermally Stable Form of Bacterial Cocaine Esterase: A Potential Therapeutic Agent for Treatment of Cocaine Abuse. Mol. Pharmacol. 77(4), 593-600.
- ↑ Narasimhan D., Nance M.R., Gao D., Ko M.C., Macdonald J., Tamburi P., Yoon D., Landry D.M., Woods J.H., Zhan C.G., Tesmer J.J., Sunahara R.K. (2009) Structural analysis of thermostabilizing mutations of cocaine esterase. Protein Eng. Des. Sel. 23:537-547.
- ↑ Benowitz NL. Clinical pharmacology and toxicology of cocaine. Pharmacol. Toxicol. 1993; 72(1): 3–12. [PubMed: 8441738]
- ↑ Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol. Rev. 1989; 41(1):3–52. [PubMed: 2682679]
- ↑ Crumb WJ Jr, Kadowitz PJ, Xu YQ, Clarkson CW. Electrocardiographic evidence for cocaine cardiotoxicity in cat. Can. J. Physiol. Pharmacol. 1990; 68(5):622–625. [PubMed: 2340451]
- ↑ Zimmerman JL. Cocaine intoxication. Crit Care Clin 2012; 28: 517–526
- ↑ Ball, JK.; Albright, V. National Estimates of Drug-Related Emergency Department Visits. Rockville, MD, USA: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2008
- ↑ A thermostable bacterial cocaine esterase rapidly eliminates cocaine from brain in nonhuman primates.LL Howell, JA Nye, JS Stehouwer, RJ Voll, J Mun, D Narasimhan, J Nichols, R Sunahara, MM Goodman, FI Carroll and JH Woods