Sandbox Reserved 996
From Proteopedia
Ectatomin (1eci) is the main component of venom of the ant Ectatomma tuberculatum, making up 15%-18% of the crude venom and accounting for 90% of the venom's toxicity.[1] When bitten by E. tuberculatum, Ectatomin inserts into the target's cell membranes and forms a nonselective cation channel.[2] The calculated isoelectric point and molecular weight of Ectatomin are 9.95 and 7928 Da, respectively.[1] While the biology regarding Ectatomin production and toxicity is not fully understood, several in vitro studies have been performed and mechanisms have been postulated.
|
Contents |
Structure
Biologically, Ectatomin exists as a heterodimer stabilized by . The has 37 amino acid residues, while the has 34 amino acid residues. The structure of Ectatomin was determined using 2D NMR and CHARMm computational optimization, though there are 20 similar proposed models in total.[3]
Generally, each subunit is composed of two antiparallel α-helices, linked by disulfide bonds, with a connecting hairpin hinge region. The two subunits are linked by a disulfide bond between their hairpin hinge regions. One α-helix from each subunit is kinked approximately 40°, due to the presence of . The kinked α-helix of the α subunit is more kinked, containing three proline residues, while the kinked α-helix of the β subunit only contains one proline residue.[3]
The internal region between the two subunits is primarily composed of hydrophobic residues.
Sequence - α subunit - GVIPKKIWETVCPTVEPWAKKCSGDIATYIKRECGKL[2]
Sequence - β subunit - WSTIVKLTICPTLKSMAKKCEGSIATMIKKKCDK[2]
Toxicology
With an LD50 of 6.8 μg kg-1, Ectatomin is one of the deadliest proteins known to man, alongside Tetanospasmin and the Botulinum toxin.[1] Ectatomin attacks and forms pores in the plasma membrane, sometimes irreversibly, allowing cations to freely diffuse through the cell membrane.[3] The primary cations which flow through the pore are Ca2+ and K+. Physiologically, the primary targets of Ectatomin are muscle, particularly the heart, and neuronal cells. As the cation concentration equilibrates between the two sides of the cell membrane, the affected cells lose their ability to form and maintain a membrane potential and concentration gradient.[2] Without the ability to form a calcium or potassium gradient, muscle cells are not able to contract and the target is rendered paralyzed. This is particularly dangerous when the heart is affected as it halts the circulatory system.[2] When neurons are affected, the inability to maintain and form a membrane potential eliminates the ability of the neuron to receive and transmit information.[4]. Either of these issues can easily result in death to the cell and the organism.
Mechanism
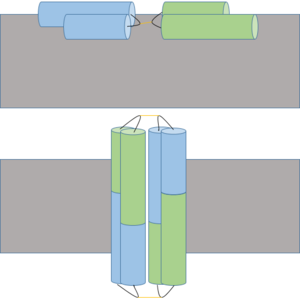
Ectatomin has several proposed mechanisms of action. The primary proposed mechanism involves the formation of a nonselective cation channel. In this mechanism, the α and β subunits open up, exposing the internal hydrophobic residues.[3] The protein flattens while remaining attached at the hairpin hinge region. The now exposed hydrophobic residues nonselectively insert into plasma membranes.[3] The inserted protein dimerizes, eventually forming a nonselective cation channel.[1]
For the second and third proposed mechanisms of action, Ectatomin has also been shown to inhibit kinases, specifically protein tyrosine kinase and protein kinase C, and natural Ca2+ channels. Kinase inhibition would allow Ectatomin to interfere with various components of signal transduction.[1] Calcium channel inhibition would allow Ectatomin to affect physiological processes such as contraction, neurotransmitter release and neuronal activity regulation.[4]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Arseniev AS, Pluzhnikov KA, Nolde DE, Sobol AG, Torgov MYu, Sukhanov SV, Grishin EV. Toxic principle of selva ant venom is a pore-forming protein transformer. FEBS Lett. 1994 Jun 27;347(2-3):112-6. PMID:8033986
- ↑ 2.0 2.1 2.2 2.3 2.4 Pluzhnikov K, Nosyreva E, Shevchenko L, Kokoz Y, Schmalz D, Hucho F, Grishin E. Analysis of ectatomin action on cell membranes. Eur J Biochem. 1999 Jun;262(2):501-6. PMID:10336635
- ↑ 3.0 3.1 3.2 3.3 3.4 Nolde DE, Sobol AG, Pluzhnikov KA, Grishin EV, Arseniev AS. Three-dimensional structure of ectatomin from Ectatomma tuberculatum ant venom. J Biomol NMR. 1995 Jan;5(1):1-13. PMID:7881269
- ↑ 4.0 4.1 Touchard A, Labriere N, Roux O, Petitclerc F, Orivel J, Escoubas P, Koh JM, Nicholson GM, Dejean A. Venom toxicity and composition in three Pseudomyrmex ant species having different nesting modes. Toxicon. 2014 Sep;88:67-76. doi: 10.1016/j.toxicon.2014.05.022. Epub 2014 Jun 11. PMID:24929139 doi:http://dx.doi.org/10.1016/j.toxicon.2014.05.022
