Sandbox WWC11
From Proteopedia
| |||||||||
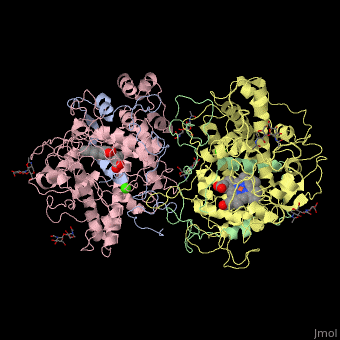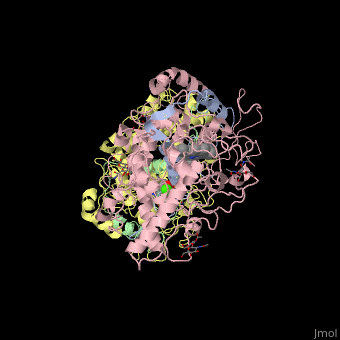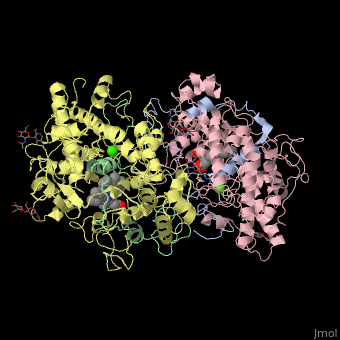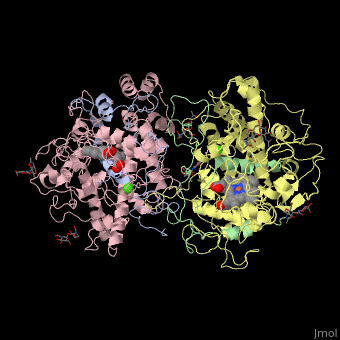
| Dog glycosylated myeloperoxidase dimer: heavy chain (pink and yellow), light chain (grey and green) containing heme complex with Ca+2 ions (green), 1D5L | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Activity: | Peroxidase, with EC number 1.11.1.7 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Introduction
Myeloperoxidase (MPO) is a protein found in neutrophil, the most common white blood cell. This enzyme generates hypochlorous acid (HOCl) from hydrogen peroxide in addition to other hypohalous acids depending on what is available in the cell. The HOCl is then used, in combination with other molecules, in an oxidative burst that kills bacteria and other potentially harmful invaders once taken up by the cell. This protein falls into a group of heme peroxidase enzymes. The role of these enzymes is to oxidize molecules using heme.[1]
This protein is composed of two identical monomers linked by a cystine bridge. The total molecular weight of this protein is 146 kDa. These two monomers can work independently of each other and consist of a light and heavy amino acid chain. The active site on the protein consists of a deep crevice where a heme molecule is covalently attached. The deep pocket is believedto limit access to the heme and increase specificity for smaller molecules to be oxidized. This pocket also possesses a hydrophobic region for substrate stabilization.[2]
A lack of or mutation in this enzyme can cause immune deficiency, although most individuals with this mutation seem to be able to compensate with other, still unknown mechanisms.[3] Over activation of myloperoxidase has been linked to increased inflammation and is being studied in relation to inceased risk of cardiovascular disease.[3] There are still many unknowns surrounding the complete function and role that myeloperoxidase plays in the body's immune response. However, it has become clear that this protein is an integral piece in the immune response and warrants further research.
Structure
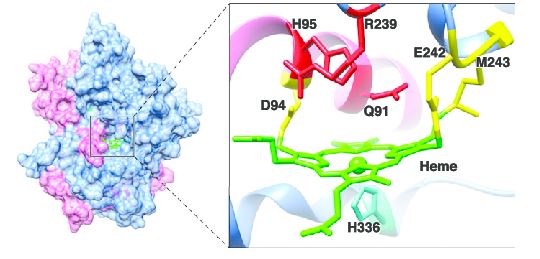
Figure 1. Depicts key amino acids in stabilization of heme in myeloperoxidase. [4]
Myeloperoxidase is synthesized in bone marrow along with most other white blood cell components. The myloperoxidase enzyme is composed of two identical subunits. After translation of these subunits, they are cleaved into two parts: the heavy chain and the light chain. The heavy chain is a glycosylated domain that weighs approximately 58.5 kDa. This portion of the enzyme has the deep pocket where the heme is inserted by chaperones (calreticulin and calnexin). [5] Click on the green link to see the in the structure above. The amino acid make up of heme pocket can be seen in Figure 1 above. The pink in this picture is the light chain, the blue is the heavy chain. The light chain (about 13.5 kDa) is attached to the heavy chain through disufide bonds. There have been discoveries of slight variation in the primary sequence of myeloperoxidase. However, for the most part these slight variations do not affect the enzymatic activity. Currently three isoforms have been isolated.[6]
Function
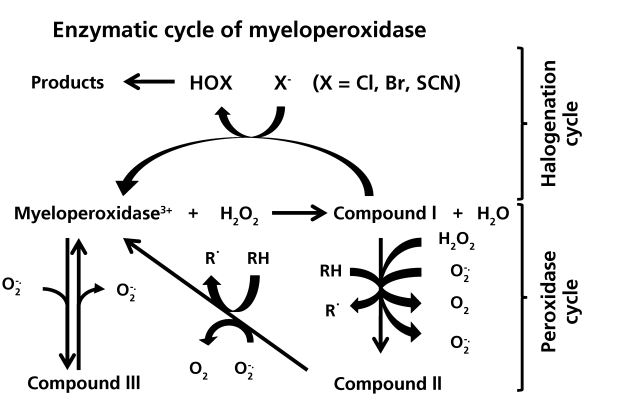
Figure 2. The Enzymatic Activity of Myeloperoxidase. [4]
As stated above, the role of the myeloperoxidase in the immune response is to generate oxidative molecules that are used to damage invaders. Seen above in Figure 2 is a concise and basic diagram of the process. Compounds I, II and III represent myeloperoxidase with different oxidation states of the iron in the heme molecule. One of the processes facilitated by myeloperoxidase is to oxidize hydrogen peroxide (H2O2) and cause the iron to change oxidation states. In this state, the iron is then able to catalyze the production of the hypohalous acids. These molecules are able to wreak havoc on lipids, proteins and DNA. [5] These modifications are achieved by the oxidation (usually by the addition of the halogen) of the unlucky recipient. The heme iron can also be oxidized by radicals to form compounds II and III. The functions of these are less well known, but the production of these radicals is also helpful in the antibacterial response.[4]
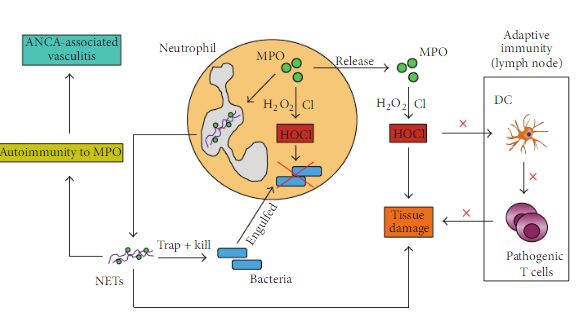
Now that the enzymatic properties of myeloperoxidase have been explored, it is time to turn to the bigger picture. These enzymes are stored in the subtype of leukocytes (white blood cells) called neutrophils. These are thought to be one of the first cells types to arrive at the site of infection.[5] An outline of the immune response of neutrophil, specifically highlighting MPO's role, can be seen in Figure 3 below. There are two paths seen in this figure. In the first path bacteria is taken up by neutrophil extracellular traps or NETs and then destroyed by the hypohalogous acids. The second path shows the MPO being released, signaled by the dendrite cells(DCs), and helping to destroy the pathogen.[5] ANCA stands for anti-neutrophil cytoplasmic antibody. The MPOs have a range of function that are steered by the neutophil cells. This intense oxidative power is very helpful when targeted to foreign bodies. However, as discussed in the next section, MPO can very destructive if released on our own tissue.
Figure 3. The immune response of neutrophils with specific emphasis on myeloperoxidase.[5]
Implication of Potential Defects or Deficiencies
Individuals with myeloperoxidase deficiency have been found to have a slight increased risk of chronic or severe infection. However, this condition is not fatal and the body seems to be able to compensate with other immune system mechanisms. [7] The most common source of MPO deficiency is ANCA (antineutrophil cytoplasmic antibodies)-associated vasculitis (AAV). This is an autoimmune disease that causes the production of antibodies for enzymes like MPO. [7] However, these diseases have also been linked to a decrease in risk of cardiovascular disease.[7]
Correlation to Cardiovascular Disease
Although MPO plays a key role in the fight against unwanted intruders, it has also been shown to turn those destructive powers against the body. Elevated levels of MPO in the blood has been found in patients with cardiovascular disease. [8] It is speculated that over activation of the immune response causes inflammation, or damage to the surrounding tissue which increases risk of cardiovascular disease. More specifically, when MPO generate the oxidative agents such as HOCl, it not only chloronate invaders, but can also chloronate lipoproteins (both LDL and HDL). This changes the lipoprotein's affinity for the cell wall which can lead to plaque buildup. It can also effects the endothelial cells lining the vein or artery.[9]
Another of the identified affects of MPO on endothelial cells is in the production of nitric oxide (NO). This is a vasodilator that is inhibited when oxidized by MPO. Myloperoxidase can also oxidize NO2 - to its radical form which has been identified as a major contributor to inflammation.[9] Because of this clear correlation between MPO activity and the manifestation of cardiovascular disease, it has the potential to be used as an indicator of a cardiovascular problem.[10]
3D structures of myeloperoxidase
Updated on 13-May-2016
1myp – MPO – dog
1mhl, 1cxp – hMPO C – human
3f9p - hMPO
1qo4 - MPO – Arabidopsis thaliana
1d2v – hMPO C + Br
1d5l – hMPO + CN
1d7w - hMPO + CN + Br
1dnu - hMPO + SCN
1dnw - hMPO + CN + SCN
3zs0, 3zs1, 4c1m - hMPO + inhibitor
4dl1 - hMPO + thioxanthine
4ejx - hMPO + ceruplasmin
References
- ↑ Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998 Nov 1;92(9):3007-17. PMID:9787133
- ↑ Yin J, Bergmann EM, Cherney MM, Lall MS, Jain RP, Vederas JC, James MN. Dual modes of modification of hepatitis A virus 3C protease by a serine-derived beta-lactone: selective crystallization and formation of a functional catalytic triad in the active site. J Mol Biol. 2005 Dec 9;354(4):854-71. Epub 2005 Oct 14. PMID:16288920 doi:10.1016/j.jmb.2005.09.074
- ↑ 3.0 3.1 Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001 Nov 7;286(17):2136-42. PMID:11694155
- ↑ 4.0 4.1 4.2 Huang J, Milton A, Arnold RD, Huang H, Smith F, Panizzi JR, Panizzi P. Methods for measuring myeloperoxidase activity toward assessing inhibitor efficacy in living systems. J Leukoc Biol. 2016 Apr;99(4):541-8. doi: 10.1189/jlb.3RU0615-256R. Epub 2016 Feb, 16. PMID:26884610 doi:http://dx.doi.org/10.1189/jlb.3RU0615-256R
- ↑ 5.0 5.1 5.2 5.3 5.4 Odobasic D, Kitching AR, Holdsworth SR. Neutrophil-Mediated Regulation of Innate and Adaptive Immunity: The Role of Myeloperoxidase. J Immunol Res. 2016;2016:2349817. doi: 10.1155/2016/2349817. Epub 2016 Jan 20. PMID:26904693 doi:http://dx.doi.org/10.1155/2016/2349817
- ↑ Cosimi AB, Shield CF, Peters C, Burton RC, Scott G, Russell PS. Prolongation of allograft survival by cyclosporin A. Surg Forum. 1979;30:287-9. PMID:120019
- ↑ 7.0 7.1 7.2 Kutter D, Devaquet P, Vanderstocken G, Paulus JM, Marchal V, Gothot A. Consequences of total and subtotal myeloperoxidase deficiency: risk or benefit ? Acta Haematol. 2000;104(1):10-5. PMID:11111115 doi:http://dx.doi.org/41062
- ↑ Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001 Nov 7;286(17):2136-42. PMID:11694155
- ↑ 9.0 9.1 Lau D, Baldus S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol Ther. 2006 Jul;111(1):16-26. Epub 2006 Feb 13. PMID:16476484 doi:http://dx.doi.org/10.1016/j.pharmthera.2005.06.023
- ↑ Brennan ML, Hazen SL. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr Opin Lipidol. 2003 Aug;14(4):353-9. PMID:12865732 doi:http://dx.doi.org/10.1097/01.mol.0000083762.66245.51