Sandbox Z-DNA BindingProteins
From Proteopedia
Contents |
Z-DNA binding proteins
|
Double Stranded RNA adenosine deaminase 1, ADAR1
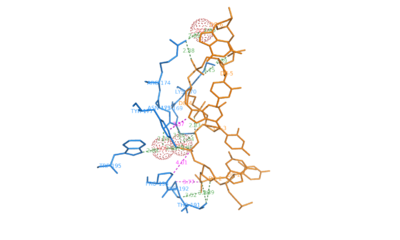
ADAR1 belongs to the family of deaminases that modify double stranded mRNA by catalyzing the conversion of adenine to inosine which is then translated to guanosine. It is a complex protein with two Z-DNA binding motifs called and Z-beta.[1] ADAR1 also has three copies of double-stranded RNA binding motif (DRBM) and a catalytic domain related to E.coli cytidine deaminase. The binding motif Z-alpha belongs to winged-helix-turn-helix family of proteins. It consists of a which has two alpha helices ( and ) connected by a short strand of amino acids and a . The beta sheet constrains the the fold by contacting the residues between alpha-2 and alpha-3.
The contact surface between consists of residues from the helix alpha-3 and COOH-terminal beta hairpin. Hydrogen bonding is present between Lys169, Lys 170, Asn173, Arg174 and Tyr177 in the helix alpha-3 and . Lys169, Asn173, Arg174, Trp195 make water mediated phosphate contacts with Z-DNA. In addition Thr191 and Arg174 of G2 and G6 on Z-DNA. An important interaction is the between aromatic ring of Tyr177 and the carbon 8 of G4. This is unique to Z-DNA as the interaction requires the base to be in syn conformation. Pro192, Pro 193 form another set of with Z-DNA where the pyrrolidine rings bond with the sugar-phosphate backbone from phosphate 2 to phosphate 3. Pro192 is conserved in Z-alpha and its homologues and forms a cis peptide bond which positions beta loop against the Z-DNA surface. [2]
The double stranded RNA substrate for ADAR1 is formed by folding of 3' intron back onto the exon containing the site to be edited. This shows that the editing of RNA occurs before the splicing of RNA providing an explanation for the binding of Z-DNA by ADAR1. Z-DNA may localize the editing activity of ADAR1 to a particular region within a gene, thus preventing indiscriminate modification. This allows for editing of the nascent transcript and blocking further transcription of gene. It has also been suggested that the extent of adenosine to inosine is proportional to amount of Z-DNA and also the ease with which the surrounding sequences adopt Z-DNA conformation. According to a study binding of ADAR1 to Z-DNA resulted in the increase in promoter activity of the gene which suggests that Z-DNA formation in the promoter region is itself involved in the regulation of transcription.[3]
Vaccinia virus E3L protein
E3L protein of vaccinia virus acts as an immune modulator and is required for replication of the virus. The region of E3L is similar to the Z-alpha domain of ADAR1 but has a lower binding affinity to Z-DNA than ADAR1 or DLM-1. Though the C-terminal of E3L is sufficient for viral replication it is the N-terminal which is responsible for pathogenicity. Mutations or deletions in the N-terminal region reduces the pathogenicity of the virus. Replacement of this domain with its corresponding analogues from ADAR1 or DLM-1 generates a chimeric virus which is as lethal as the wild type virus. Thus a drug which can block the binding of E3L to Z-DNA may be an effective therapy in preventing pathogenicity. Similarity of E3L to variola also suggests that such drugs might be effective against small pox. [1][4] `
DLM-1
DLM-1 is also known as Z-DNA binding protein 1 (ZBP1). It is expressed by a tumor associated gene in lymphatic tissues and is upregulated in the peritoneal lining of mice with mouse ovarian ascites tumor. DLM-1 has two Z-DNA binding domains analogous to the Z-alpha and Z- beta domains in ADAR1. Comparison of Z-DNA binding of DLM-1 and ADAR1 revealed a common structure recognition core within the binding domain. However the role of DLM-1 binding to Z-DNA in tumor development is not known.
Z-DNA binding proteins have common structural characteristics. The binding domains of these proteins can substitute one another and thus can act as competitive inhibitors against one another. As explained above, disruption in the Z-DNA binding region of E3L reduces its pathogenicity. All these observations are important pointers towards the biological importance of Z-DNA.[1]
Movie Depicting ADAR1 binding to Z-DNA
References
- ↑ 1.0 1.1 1.2 Wang G, Vasquez KM. Z-DNA, an active element in the genome. Front Biosci. 2007 May 1;12:4424-38. PMID:17485386
- ↑ Schwartz T, Rould MA, Lowenhaupt K, Herbert A, Rich A. Crystal structure of the Zalpha domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA. Science. 1999 Jun 11;284(5421):1841-5. PMID:10364558
- ↑ Rich A, Zhang S. Timeline: Z-DNA: the long road to biological function. Nat Rev Genet. 2003 Jul;4(7):566-72. PMID:12838348 doi:10.1038/nrg1115
- ↑ Kim YG, Lowenhaupt K, Oh DB, Kim KK, Rich A. Evidence that vaccinia virulence factor E3L binds to Z-DNA in vivo: Implications for development of a therapy for poxvirus infection. Proc Natl Acad Sci U S A. 2004 Feb 10;101(6):1514-8. Epub 2004 Feb 2. PMID:14757814 doi:10.1073/pnas.0308260100