Sandbox vdr
From Proteopedia
Contents |
Vitamin D Receptor
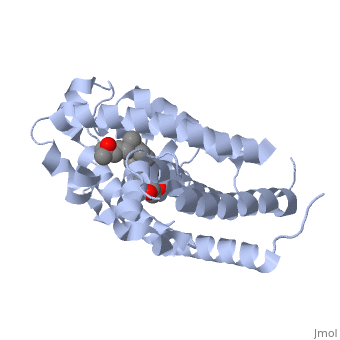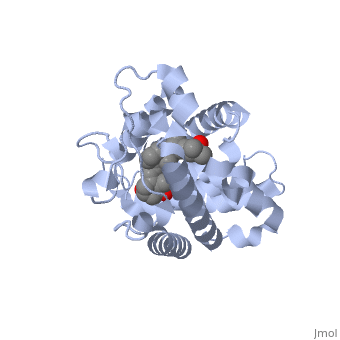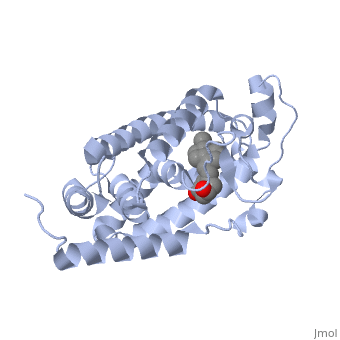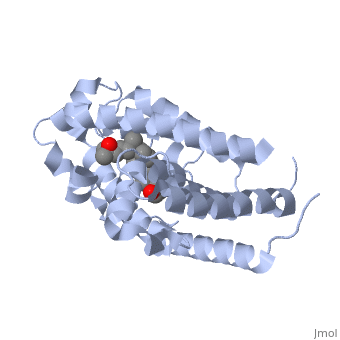
Vitamin D receptor () is a transcription factor. Upon binding to vitamin D, VDR forms a heterodimer with retinoid-X receptor and binds to hormone response receptors on DNA causing gene expression. The (in green) binds to receptors in its target cells, controlling the synthesis of many different proteins involved in calcium transport and utilization. VDR contains two domains: a that binds to the hormone (grey) and that binds to DNA. (Green and blue are two same VDR structures). It pairs up with a similar protein, 9-cis retinoic acid receptor (RXR), and together they bind to the DNA, activating synthesis in some cases and repressing it in others.
Crystal Structure of the nuclear receptor for Vitamnin D complexed to Vitamin D
The action of 1 alpha, 25-dihydroxyvitamin D3 is mediated by its nuclear receptor (VDR), a ligand-dependent transcription regulator. We report the 1.8 A resolution crystal structure of the complex between a VDR ligand-binding domain (LBD) construct lacking the highly variable VDR-specific insertion domain and vitamin D. The construct exhibits the same binding affinity for vitamin D and transactivation ability as the wild-type protein, showing that the N-terminal part of the LBD is essential for its structural and functional integrity while the large insertion peptide is dispensable. The structure reveals the active conformation of the bound ligand and allows understanding of the different binding properties of some synthetic analogs.
The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand., Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D, Mol Cell. 2000 Jan;5(1):173-9. PMID:10678179
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
Disease
[VDR_HUMAN] Defects in VDR are the cause of rickets vitamin D-dependent type 2A (VDDR2A) [MIM:277440]. A disorder of vitamin D metabolism resulting in severe rickets, hypocalcemia and secondary hyperparathyroidism. Most patients have total alopecia in addition to rickets.[1][2][3][4][5][6][7][8][9][10]
Function
Vitamin D plays an essential role in regulating the levels of calcium and phosphate in the body. It is converted into a hormone that is secreted by the kidneys and travels through the body. It has major effects on intestinal cells, where it helps control the uptake of calcium, and bone cells, where it helps control the formation and maintenance of the skeleton.
[VDR_HUMAN] Nuclear hormone receptor: Transcription factor that mediates the action of vitamin D3 by controlling the expression of hormone sensitive genes. Regulates transcription of hormone sensitive genes via its association with the WINAC complex, a chromatin-remodeling complex. Recruited to promoters via its interaction with the WINAC complex subunit BAZ1B/WSTF, which mediates the interaction with acetylated histones, an essential step for VDR-promoter association. Plays a central role in calcium homeostasis.[11][12][13][14]
Mutation
| |||||||||||
Crystal structure of the human VDR ligand binding domain bound to the synthetic agonist compound 2alpha-methyl-AMCR277A(C23S)
| |||||||||||
About this Structure
1db1 is a 1 chain structure with sequence from Homo sapiens. Full crystallographic information is available from OCA. On right hand side is Structure of human vitamin D receptor ligand-binding domain complex with vitamin D (PDB entry 1db1).
See Also
3D Structures of vitamin D receptor
Updated on 21-November-2013
Vitamin D receptor ligand-binding domain
3m7r - hVDR LBD (mutant) – human
1db1 – hVDR LBD + vitamin D
1s0z, 1s19, 3a78, 4g2i - hVDR LBD + vitamin D derivative
3ogt, 2ham, 2har, 2has, 2hb7, 2hb8, 3p8x, 3az1, 3az2, 3az3, 3tkc - hVDR LBD + vitamin D analog
3auq, 3aur, 3kpz - hVDR LBD (mutant) + vitamin D analog
3a2i, 3a2j, 3b0t, 3ax8, 3vhw - hVDR LBD (mutant) + vitamin D derivative
3a3z, 3a40 - hVDR LBD + agonist
1ie8, 1ie9, 3cs4, 3cs6, 1txi – hVDR LBD + superagonist
3w5q, 3w5r, 3w5t - hVDR LBD + lithocholic acid derivative
Vitamin D receptor LBD complex with peptide
1rjk, 1rk3, 1rkg, 1rkh, 2o4j, 2o4r – rVDR LBD (mutant) + peroxisome proliferator-activated receptor peptide – rat
2zl9, 2zla, 2zlc - rVDR LBD + coactivator peptide DRIP + vitamin D analog
3vrt, 3vru, 3vrv, 3vrw - rVDR LBD (mutant) + coactivator peptide DRIP + vitamin D analog
2zfx, 3a2h, 2zxm, 2zxn - rVDR LBD + coactivator peptide DRIP
3aun, 2zmh, 2zmi, 2zmj, 3afr, 3vjs, 3vjt - rVDR LBD (mutsant) + coactivator peptide DRIP
2hbh - zVDR LBD + steroid receptor coactivator 1 peptide – zebrafish
2hc4 - zVDR LBD + steroid receptor coactivator 1 peptide + vitamin D
2hcd - zVDR LBD + steroid receptor coactivator 1 peptide
Vitamin D receptor DNA-binding domain
1kb2 – hVDR DBD + osteopontin response element DNA
1kb4 – hVDR DBD + DR3 response element DNA
1ynw – hVDR DBD (mutant) + DR3 response element DNA
1kb6 – hVDR DBD + osteocalcin response element DNA
</StructureSection>
Reference
- Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell. 2000 Jan;5(1):173-9. PMID:10678179
- Tocchini-Valentini G, Rochel N, Wurtz JM, Mitschler A, Moras D. Crystal structures of the vitamin D receptor complexed to superagonist 20-epi ligands. Proc Natl Acad Sci U S A. 2001 May 8;98(10):5491-6. PMID:11344298 doi:10.1073/pnas.091018698
- ↑ Hughes MR, Malloy PJ, Kieback DG, Kesterson RA, Pike JW, Feldman D, O'Malley BW. Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science. 1988 Dec 23;242(4886):1702-5. PMID:2849209
- ↑ Yagi H, Ozono K, Miyake H, Nagashima K, Kuroume T, Pike JW. A new point mutation in the deoxyribonucleic acid-binding domain of the vitamin D receptor in a kindred with hereditary 1,25-dihydroxyvitamin D-resistant rickets. J Clin Endocrinol Metab. 1993 Feb;76(2):509-12. PMID:8381803
- ↑ Saijo T, Ito M, Takeda E, Huq AH, Naito E, Yokota I, Sone T, Pike JW, Kuroda Y. A unique mutation in the vitamin D receptor gene in three Japanese patients with vitamin D-dependent rickets type II: utility of single-strand conformation polymorphism analysis for heterozygous carrier detection. Am J Hum Genet. 1991 Sep;49(3):668-73. PMID:1652893
- ↑ Sone T, Marx SJ, Liberman UA, Pike JW. A unique point mutation in the human vitamin D receptor chromosomal gene confers hereditary resistance to 1,25-dihydroxyvitamin D3. Mol Endocrinol. 1990 Apr;4(4):623-31. PMID:2177843
- ↑ Malloy PJ, Weisman Y, Feldman D. Hereditary 1 alpha,25-dihydroxyvitamin D-resistant rickets resulting from a mutation in the vitamin D receptor deoxyribonucleic acid-binding domain. J Clin Endocrinol Metab. 1994 Feb;78(2):313-6. PMID:8106618
- ↑ Kristjansson K, Rut AR, Hewison M, O'Riordan JL, Hughes MR. Two mutations in the hormone binding domain of the vitamin D receptor cause tissue resistance to 1,25 dihydroxyvitamin D3. J Clin Invest. 1993 Jul;92(1):12-6. PMID:8392085 doi:http://dx.doi.org/10.1172/JCI116539
- ↑ Rut AR, Hewison M, Kristjansson K, Luisi B, Hughes MR, O'Riordan JL. Two mutations causing vitamin D resistant rickets: modelling on the basis of steroid hormone receptor DNA-binding domain crystal structures. Clin Endocrinol (Oxf). 1994 Nov;41(5):581-90. PMID:7828346
- ↑ Lin NU, Malloy PJ, Sakati N, al-Ashwal A, Feldman D. A novel mutation in the deoxyribonucleic acid-binding domain of the vitamin D receptor causes hereditary 1,25-dihydroxyvitamin D-resistant rickets. J Clin Endocrinol Metab. 1996 Jul;81(7):2564-9. PMID:8675579
- ↑ Whitfield GK, Selznick SH, Haussler CA, Hsieh JC, Galligan MA, Jurutka PW, Thompson PD, Lee SM, Zerwekh JE, Haussler MR. Vitamin D receptors from patients with resistance to 1,25-dihydroxyvitamin D3: point mutations confer reduced transactivation in response to ligand and impaired interaction with the retinoid X receptor heterodimeric partner. Mol Endocrinol. 1996 Dec;10(12):1617-31. PMID:8961271
- ↑ Malloy PJ, Eccleshall TR, Gross C, Van Maldergem L, Bouillon R, Feldman D. Hereditary vitamin D resistant rickets caused by a novel mutation in the vitamin D receptor that results in decreased affinity for hormone and cellular hyporesponsiveness. J Clin Invest. 1997 Jan 15;99(2):297-304. PMID:9005998 doi:10.1172/JCI119158
- ↑ Fujiki R, Kim MS, Sasaki Y, Yoshimura K, Kitagawa H, Kato S. Ligand-induced transrepression by VDR through association of WSTF with acetylated histones. EMBO J. 2005 Nov 16;24(22):3881-94. Epub 2005 Oct 27. PMID:16252006 doi:10.1038/sj.emboj.7600853
- ↑ Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell. 2000 Jan;5(1):173-9. PMID:10678179
- ↑ Eelen G, Verlinden L, Rochel N, Claessens F, De Clercq P, Vandewalle M, Tocchini-Valentini G, Moras D, Bouillon R, Verstuyf A. Superagonistic action of 14-epi-analogs of 1,25-dihydroxyvitamin D explained by vitamin D receptor-coactivator interaction. Mol Pharmacol. 2005 May;67(5):1566-73. Epub 2005 Feb 22. PMID:15728261 doi:10.1124/mol.104.008730
- ↑ Hourai S, Fujishima T, Kittaka A, Suhara Y, Takayama H, Rochel N, Moras D. Probing a water channel near the A-ring of receptor-bound 1 alpha,25-dihydroxyvitamin D3 with selected 2 alpha-substituted analogues. J Med Chem. 2006 Aug 24;49(17):5199-205. PMID:16913708 doi:http://dx.doi.org/10.1021/jm0604070