Investigating the Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD)
From Proteopedia
Contents |
Investigating the Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD) [1]
The following article is a product of the Intro to Protein Modeling course offered by the Farquhar Honors College from Nova Southeastern University, under the direction of Dr. Arthur Sikora and Dr. Emily Schmitt Lavin.
The research question that was sought to be answered: How do specific human Medium Acyl-CoA Dehydrogenase (MCAD) enzyme mutations contribute to Medium Acyl-CoA Dehydrogenase Deficiency (MCADD)?
Abstract
Medium-chain Acyl-CoA Dehydrogenase Deficiency (MCADD) is a human disorder that hinders β-oxidation, affecting approximately 1 in 17,000 people in the United States.[2] Once mutated, the Acyl-CoA Dehydrogenase Medium-Chain (ACADM) gene, which is solely responsible for MCADD, cannot produce enough MCAD enzymes to metabolize medium-chain fatty acids. As a result, fats are not catabolized, causing symptoms of lethargy and hypoglycemia, as well as damage to the brain and liver due to a buildup of unused fatty tissue. The purpose of this project was to investigate the possible and known effects of different amino acid mutations on the human MCAD protein and produce a 3D-printed model to explain the molecular story of MCADD. This model builds on previous bioinformatics and in vivo experiments aimed at revealing the underlying enzymatic mechanisms of MCADD. Using PyMOL, the human wild-type MCAD (PDB ID: 1T9G) had its electron-transferring flavoprotein (ETF) complex removed and a single chain from its homotetramer portion isolated for clarity. PyRx was used to dock the substrate, Octanoyl-CoA (PDB ID: CO8) into the slightly mutated enzyme, referencing PDB ID 1EGC. Known mutations from the PDB files and related literature were then compared and analyzed on the modified 1T9G to determine known and possible effects the mutations had, such as helix-helix stability and ligand hydrogen bonding. LigPlot+ was then used to analyze ligand-active site interactions. Jmol was used to cosmetically enhance the modified 1T9G to produce a 3D model for printing. In the model, the mutations were ranked according to known KM range values (0.4, 0.6), (0.7, 0.9), and 0.9+, which were highlighted in the colors “lightskyblue”, “royalblue”, and “midnightblue”, respectively; unknown KM values were colored “chartreuse”. All mutations had their side chains shown for further clarity. E376, the catalytic base, was colored “magenta”, the backbone was colored “dimgray”, and the support struts of the model were colored “lightseagreen”. The use of the 3D model was beneficial, enabling model viewers to locate, determine, and hypothesize the mutations and their effects on MCAD, in addition to providing a visual and physical learning aid for researchers, professors, students, and other biomedical professionals. Furthermore, the clarity produced by a physical model ultimately enables further research for MCADD and may assist in the development of a cure for those who unfortunately suffer from this rare condition.
Introduction
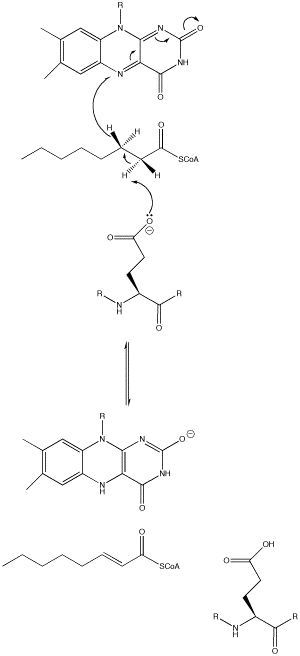
An important enzyme in β-oxidation is Acyl-CoA Dehydrogenase, which abstracts a hydrogen atom from its fatty acyl-CoA substrate and inserts it on FAD, an electron carrier. With FAD also removing a fatty acyl-CoA hydrogen, FAD is reduced to FADH2, which is utilized in the electron transport chain to ultimately produce ATP, forming a double bond on the acyl-CoA chain. The biochemical mechanism is shown in the image below.[3] In Medium Acyl-CoA Dehydrogenase Deficiency (MCADD), mutations in the ACADM (Acyl-CoA Dehydrogenase Medium-Chain) gene, the only gene that causes MCADD, render less functional MCADs.[4] Since MCADD is the most common defect in the pathway of β-oxidation, and MCAD (medium-chain acyl-CoA dehydrogenase) is needed to metabolize medium-chain fatty acids, a deficiency of this protein has effects ranging from hypoglycemia and lethargy, and damage to the brain and liver due to a buildup of fatty tissue.[4] Understanding of the mutations that caused the disease was sought; amino acid mutations that overlapped across the studies researched and were able to be visualized in the Human WT MCAD (PDB ID: 1T9G) were recorded and analyzed for their effects on the protein (i.e., helix-helix interactions, H-bonding to ligand) and how it could contribute to MCAD; these mutations are listed in Table 1.
Materials & Methods
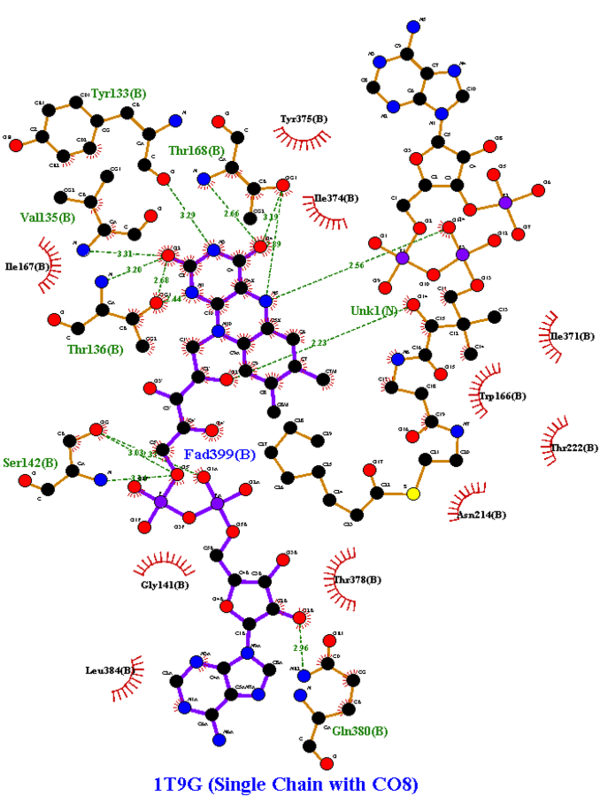
From the Protein Data Bank, the human WT MCAD (PDB ID: 1T9G) was collected, shown below.[5] Multiple articles were researched for various mutations, which were analyzed from the article and viewed in PyMOL.[6][7][8] Additional computerized modifications were needed, however. Using PyMOL, the electron-transferring flavoprotein (ETF) complex of 1T9G was removed, and chain B was isolated from the MCAD homotetramer portion for better focus. No substrate was on 1T9G originally, so Octanoyl-CoA (PDB ID: CO8) was docked using PyRx; CO8 was a ligand in the protein 1EGC, a similar yet slightly mutated version of human MCAD used for reference. LigPlot+ was then used to identify the amino acids that undergo hydrogen bonding and hydrophobic interactions with FAD & CO8. Finally, the modified 1T9G was cosmetically enhanced in Jmol to produce a detailed 3D model.
|
Note: to view the unmodified 1T9G again, you must refresh the page.
Results
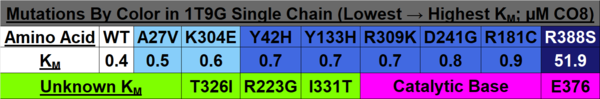
To make the model more understandable, the mutations were grouped and color-coded based on the Table below:
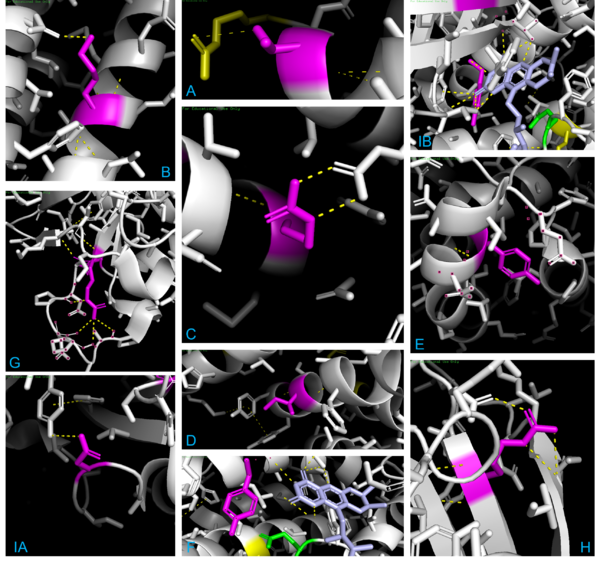
LigPlot+ was used to analyze and determine the active site of the modified 1T9G protein:
The ultimate result of this project (and course) was to create a 3D model in Jmol, shown in detail below:
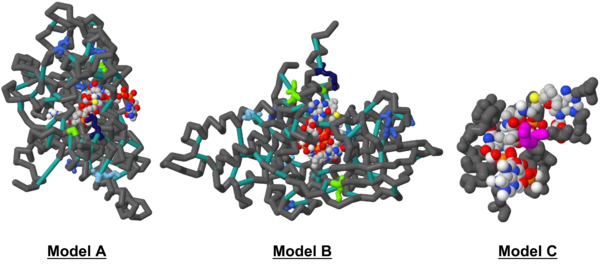
The modified 1T9G PDB file can be viewed under the Materials & Methods section.
Discussion
When observing 1T9G in PyMOL (see Figure 4 below), there are 3 arginine residues (R256, R324, & R388) that interact with CO8, so R388S would greatly lessen ligand interaction, explaining the high KM outlier. T326 interacted with R324, so although the KM value is unknown, it is predicted that the KM for T326I would lie within a high range, as it was found to lead to aggregation and thus a loss of function. K304 & R309 impact helix-helix stability via interactions with Q342 & E85, respectively; thus, mutations would destabilize them, explaining R309K’s higher KM. I331T would destabilize two α-helices by disrupting a series of π-interactions; while its KM is unknown, it is predicted that it lies within the intermediate/high range described previously, as it led to a complete loss of protein function due to aggregation. Y133 maps to the β-sheet domain, directly H-bonding with FAD along with the residues T136 & T168; Y133H might interact with E376 and impair helix-helix stability, explaining its intermediate KM. D241 stabilizes a β-sheet whose end residue bonds to FAD, explaining its high KM. R223G would unlink β-sheet interactions; while its KM is unknown, it was predicted to be within the higher range as it was shown to have a complete loss of function due to aggregation, just like T326I & I331T. R181, while far from the active site, makes four bonds with other amino acids, thus explaining its second-highest KM when mutated. A27V is mostly unaffected regarding both size & charge, explaining its KM similarity to the WT. Y42H might cause an interaction with nearby E41 & E47, explaining its lower intermediate KM. The 3D model helps visualize these mutations and their effects.
Applications
3D protein modeling applications include visual representations and kinesthetic movement of molecular processes and enriching education in biological, biochemical, and other related classes. This specific project best applies to assisting clinical researchers in further understanding the biochemical causes and effects of the MCAD mutations, as well as their severity.
Acknowledgments
This work was made possible by funding through the Division of Undergraduate Education at the National Science Foundation (NSF-DUE) grant number 1725940 for the CREST Project. Nova Southeastern University’s Farquhar Honors College and Dept. of Biological Sciences also provided support. Protein model printing was made possible by 3d Molecular Designs. We also extend our gratitude to those who published PDBs 1T9G & 1EGC, as well as those who provided information on the MCAD amino acid mutations and ACADM nucleotide mutations.
References
- ↑ Saleh, Omar E.; Khatiwala, Rhea; and Ignatius, Jeremy, "Investigating The Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD)" (2022). Protein Modeling Reports. 7. https://nsuworks.nova.edu/protein_modeling_reports/7
- ↑ https://medlineplus.gov/genetics/condition/medium-chain-acyl-coa-dehydrogenase-deficiency/
- ↑ 3.0 3.1 https://commons.wikimedia.org/wiki/File:AcylCoAdehydrogenase.png
- ↑ 4.0 4.1 Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090
- ↑ Toogood, H. S., van Thiel, A., Basran, J., Sutcliffe, M. J., Scrutton, N. S., & Leys, D. (2004) Extensive domain motion and electron transfer in the human electron transferring flavoprotein·medium chain acyl-COA dehydrogenase complex. Journal of Biological Chemistry, 279(31), 32904–32912. https://doi.org/10.1074/jbc.m404884200
- ↑ Maier, E. M., Gersting, S. W., Kemter, K. F., Jank, J. M., Reindl, M., Messing, D. D., Truger, M. S., Sommerhoff, C. P., & Muntau, A. C. (2009). Protein misfolding is the molecular mechanism underlying MCADD identified in newborn screening. Human molecular genetics, 18(9), 1612–1623. https://doi.org/10.1093/hmg/ddp079
- ↑ McAndrew, R. P., Wang, Y., Mohsen, A. W., He, M., Vockley, J., & Kim, J. J. (2008). Structural basis for substrate fatty acyl chain specificity: crystal structure of human very-long-chain acyl-CoA dehydrogenase. The Journal of biological chemistry, 283(14), 9435–9443. https://doi.org/10.1074/jbc.M709135200
- ↑ Tucci, S., Wagner, C., Grünert, S. C., Matysiak, U., Weinhold, N., Klein, J., Porta, F., Spada, M., Bordugo, A., Rodella, G., Furlan, F., Sajeva, A., Menni, F., & Spiekerkoetter, U. (2021). Genotype and residual enzyme activity in medium-chain acyl-CoA dehydrogenase (MCAD) deficiency: Are predictions possible? Journal of inherited metabolic disease, 44(4), 916–925. https://doi.org/10.1002/jimd.12368