Arabidopsis thaliana PIN-FORMED 3 (AtPIN3)
From Proteopedia
| Line 17: | Line 17: | ||
The helices 1, 2 and 7 of the scaffold domains are involved in dimerization through symmetric interactions with a surface area of 1516 Å. The tilted TM7 interacts with TM 1, TM2 and TM7 of the other subunit through hydrophobic packaging. TM2 establishes hydrophobic packaging at the base of the dimer. This combined scaffold domain remains static and has the transporter domain on either side of it. | The helices 1, 2 and 7 of the scaffold domains are involved in dimerization through symmetric interactions with a surface area of 1516 Å. The tilted TM7 interacts with TM 1, TM2 and TM7 of the other subunit through hydrophobic packaging. TM2 establishes hydrophobic packaging at the base of the dimer. This combined scaffold domain remains static and has the transporter domain on either side of it. | ||
| - | The transport domain is predicted to undergo up-down rigid-body motion in an elevator-like model (Figure 3). Two weak helices TM4 and TM9 break in the middle and cross and connect to each other as short loops. These may provide a substrate binding site and allow for confirmational changes during auxin transport. A solvent accessible pathway is present between the scaffold and the transport domain. This was suggested as the location for the <scene name='10/1096831/Iaa_bound_state/ | + | The transport domain is predicted to undergo up-down rigid-body motion in an elevator-like model (Figure 3). Two weak helices TM4 and TM9 break in the middle and cross and connect to each other as short loops. These may provide a substrate binding site and allow for confirmational changes during auxin transport. A solvent accessible pathway is present between the scaffold and the transport domain. This was suggested as the location for the <scene name='10/1096831/Iaa_bound_state/2'>binding of IAA</scene>. The elevator model is supported by a structural alignment of PIN3 in its apo and IAA bound state, which shows a movement of the transport domain 2-3 Å towards the scaffold domain once AA binds. |
| - | The protein has a cytosolic domain which contains the cytosolic extension of TM5, | + | The protein has a cytosolic domain which contains the cytosolic extension of TM5, .The loop between the AH and β3 has many phosphorylation sites that regulate the subcellular localization and transport activity of the protein. |
| - | + | an Amphiphilic helix (AH) and 3 beta sheets | |
== Green links to be moved == | == Green links to be moved == | ||
| - | + | <scene name='10/1096831/Cytosolic_ah_and_beta-strand/1'>an Amphiphilic helix (AH) and 3 beta sheets (β1–3)</scene> | |
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 10:53, 30 November 2025
Contents |
AtPIN3
| |||||||||||
Function
Auxin and hence the PIN proteins are involved in many processes like embryogenesis, organogenesis, cell fate determination, and cell division. It also contributes to trophic responses like gravitropism and phototropism.
Mutation of PIN genes or their improper localization may lead to many developmental defects like shorter roots, reduced number of lateral roots, root meristem collapse, defective columella cells, abnormal cotyledons and altered leaf venation
Structure
AtPIN3 is its is a homodimer with 10 transmembrane (TM1-TM10) domains each Figure 2. Both the N and the C terminal of the protein lie on the extracellular side. The 10 TM domains are divided into two groups a scaffold domain (TM1–2 and 6–7) and a transport domain (TM3–5 and 8–10).
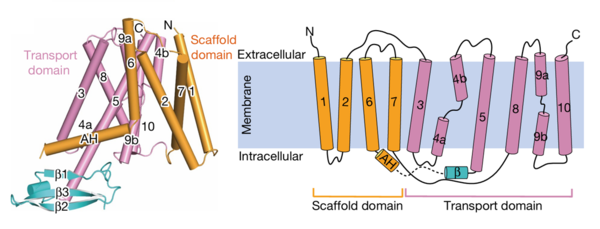
The helices 1, 2 and 7 of the scaffold domains are involved in dimerization through symmetric interactions with a surface area of 1516 Å. The tilted TM7 interacts with TM 1, TM2 and TM7 of the other subunit through hydrophobic packaging. TM2 establishes hydrophobic packaging at the base of the dimer. This combined scaffold domain remains static and has the transporter domain on either side of it.
The transport domain is predicted to undergo up-down rigid-body motion in an elevator-like model (Figure 3). Two weak helices TM4 and TM9 break in the middle and cross and connect to each other as short loops. These may provide a substrate binding site and allow for confirmational changes during auxin transport. A solvent accessible pathway is present between the scaffold and the transport domain. This was suggested as the location for the . The elevator model is supported by a structural alignment of PIN3 in its apo and IAA bound state, which shows a movement of the transport domain 2-3 Å towards the scaffold domain once AA binds.
The protein has a cytosolic domain which contains the cytosolic extension of TM5, .The loop between the AH and β3 has many phosphorylation sites that regulate the subcellular localization and transport activity of the protein.
an Amphiphilic helix (AH) and 3 beta sheets
Green links to be moved
References
- ↑ Su, N., Zhu, A., Tao, X. et al. Structures and mechanisms of the Arabidopsis auxin transporter PIN3. Nature 609, 616–621 (2022).DOI:https://doi.org/10.1038/s41586-022-05142-w
![Figure 1: Auxin (IAAH) enters the cell through influx transporter passes directly through the plasma membrane. Auxin dissociates to release a proton (H+) and anion (IAA-) in the cytoplasm due to higher pH. Due to its charge, it requires PIN proteins to carry it out of the cell. Once it reenters the apoplast it can bind to H again and it moves to the next cell Image by Jen Valenzuela [(CC-BY-NC)]](/wiki/images/thumb/7/74/PIN3.png/400px-PIN3.png)
