User:Luis E Ramirez-Tapia/Sandbox 1
From Proteopedia
Contents |
What is a Helicase?
| |||||||||
| 1jpr, resolution 1.88Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Activity: | Ribonucleoside-diphosphate reductase, with EC number 1.17.4.1 | ||||||||
| Related: | 1jqc | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Helicases are nucleic acid–dependent ATP-ases that are capable of unwinding DNA [1] or RNA [2] duplex substrates. As a consequence, they play roles in almost every process in cells that involves nucleic acids, including DNA replication and repair, transcription, translation, ribosome synthesis (1). PcrA is part of the helicase superfamily I. A monomeric protein that is mainly alfa helical
Repressors are proteins that inhibit the expression of DNA]; that is, they inhibit the transcription of messenger RNA from their target genes. Each repressor targets a specific co-regulated group of genes by recognizing a specific sequence of DNA, called the operator in bacteria. Repressor proteins are coded for by regulatory genes.
The lactose ("lac") repressor controls the expression of bacterial enzymes involved in the metabolism of of the sugar lactose. When the lac repressor binds lactose, it changes to an inactive conformation that cannot repress the production of these enzymes. Thus, the enzymes needed to use lactose are made only when lactose is available. The lac repressor, and the group of genes it controls, which is called an operon, were the first such gene regulatory system to be discovered. The operon was described in 1960[1] by François Jacob et al., who also correctly proposed the general mechanism of regulation by the lac repressor. The 1965 Nobel Prize in Physiology or Medicine was awarded to François Jacob, André Lwoff, and Jacques Monod "for their discoveries concerning genetic control of enzyme and virus synthesis".
For a general introduction to the lac repressor, please see David Goodsell's Introduction to the lac repressor in his series Molecule of the Month, and the article in Wikipedia on the lac repressor. Mitchell Lewis published a detailed review in 2005[2].
Structure of the lac repressor
|
The lac repressor protein ( showing chain A in 1lbg, resolution 4.8 Å), starting at the N-terminus, begins with a DNA-binding "headpiece", followed by a hinge region, then an N-terminal ligand-binding subdomain and a C-terminal ligand binding subdomain, a linker, and a C-terminal tetramerization helix[3]. (.) In the absence of DNA, the hinge region does not form the alpha helix shown here.
As can be seen when the chain is
| N | C |
each of the ligand-binding subdomains is made up of two discontinuous segments.
The lac repressor forms . Dimerization buries 2,200 Å2 of surface, including a ,
forming a hydrophobic core (shown with 1lbi, resolution 2.7 Å, lacking the DNA-binding domain due to disorder).
 |
The most highly is the surface that contacts DNA[4]. (Only alpha carbon atoms are shown here, without sidechains, because sidechains were not resolved in the 4.8 Å 1lbg model.) The dimerization surfaces are the of the ligand-binding domains[5]. (This scene shows sidechains, using the 2.7 Å model in 1lbi, which lacks the DNA-binding domain due to disorder.)
<--! scene with translucent :a is #11, but I didn't like it. --> The C-terminal tetramerization helices tether two dimers, and thus the functional form of with two DNA-binding sites.
DNA Binding: Bending the Operator
Non-Specific Binding
|
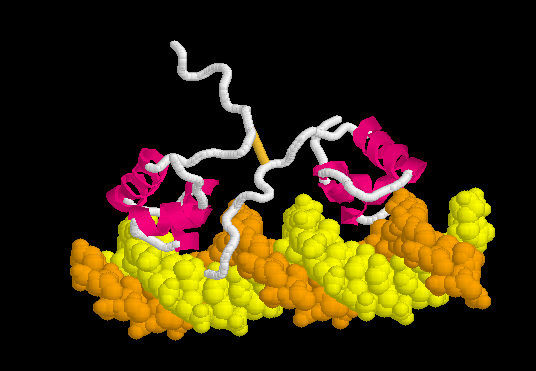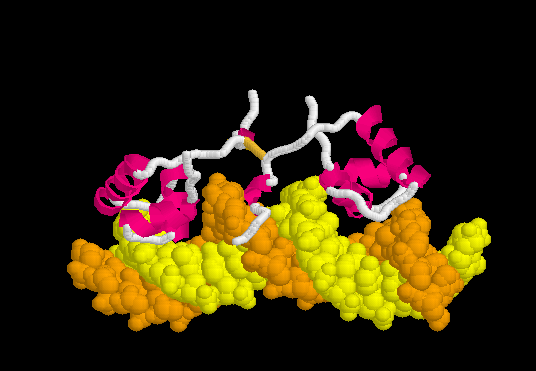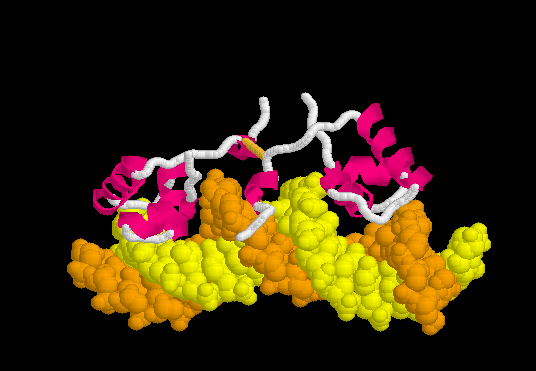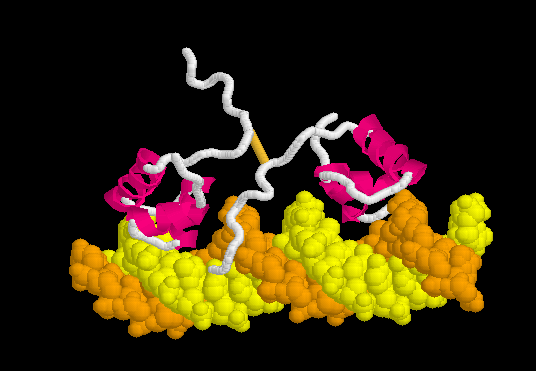
Lac repressor binds to DNA non-specifically ( derived [6] from 1osl, 20 NMR models), enabling it to slide rapidly along the DNA double helix until it encounters the lac operator sequence. The DNA-binding domain employs a helix-turn-helix motif (Alpha Helices, Turns). During non-specific binding, the hinge region is disordered (indicated by the range of positions of the 20 models), and the DNA double helix is straight. The model shown at right (1osl) has two copies of the DNA-binding domain and hinge region ( to distinguish the chain B hinge). these 20 NMR models simulates thermal motion of the disordered hinge regions.
Specific Binding
Upon recognizing the specific operator sequence, the non-specific binding converts to (derived[6] from 1l1m, 20 NMR models). During this conversion, the hinge region changes from disordered loops to Alpha Helices (), which bind in the minor groove of the DNA. This binding opens the minor groove, bending the DNA double helix. these can be compared with the animation of the non-specific binding.
Morph of Conversion
The can be seen more easily when they are animated smoothly by morphing. (The methods used to create this morph are given in Lac repressor morph methods.) Note the bending of the DNA, with the widening of the central minor groove on the convex aspect. Also note the conversion of the disulfide-bonded hinge region loops to alpha helices. (The displayed secondary structure is calculated for each model in the morph interpolation.)
The specific recognition of the lac operator sequence in the DNA occurs largely though hydrogen bonds. is illustrated in this rendering of the morph. Shown are hydrogen bonds involving Arg22.N-eta2 and Tyr18.OH interacting with DNA base oxygens in the major groove, and Ala53.O interacting with a DNA base nitrogen in the minor groove. (Not all of the relevant hydrogen bonds are shown; see Methods.)
Test:
Animation for Powerpoint® Slides
Here is an animated multi-gif true movie of the above morph, ready to insert into a Powerpoint®[7] slide.
- In Windows, simply drag the movie and drop it into the Powerpoint slide. You can then resize it and position it. The movie should play when you change the View to Slide Show ("project") the slide.
- In Mac OSX, Ctrl-Click on the movie, then Save Image. In Mac Powerpoint, at the desired slide, use the Insert menu (at the top) and select Movie ..., then insert the saved .gif movie file. After inserting the movie, make sure the Toolbox is showing (controlled with an icon-button at the top of the window). Now you can resize and reposition the movie. Click in the movie in the slide to select it. Now, in the Toolbox/Formatting Palette, under Movie, check Loop Until Stopped. Now the movie should play when you change the View to Slide Show ("project") the slide.
Challenge Your Understanding
Here are some questions to challenge your understanding.
- Why does the lac repressor bind to DNA non-specifically?
- When the lac repressor binds non-specifically to DNA, what part of the DNA double helix does it bind to?
- Does DNA have a net charge, and if so, is it negative or positive in aqueous solution at pH 7?
- What kinds of chemical bonds are likely to be involved in non-specific binding of the repressor protein to DNA?
- Does specific binding of lac repressor to DNA disrupt any of the Watson-Crick hydrogen bonds between the base pairs in the DNA strands?
- How do proteins such as the lac repressor recognize specific nucleotide sequences in a DNA double helix?
- What kinds of chemical bonds are involved in specific binding of the repressor protein to DNA?
- Does the lac repressor recognize specific bases in the major or minor grooves of the DNA?
- Why does the lac repressor bend the DNA double helix when it recognizes its specific nucleotide sequence?
Answers are available on request to ![]() . If you would like us to make the answers publically available within Proteopedia, please let us know. When contacting us, please give your full name, your position, institution or school, and location.
. If you would like us to make the answers publically available within Proteopedia, please let us know. When contacting us, please give your full name, your position, institution or school, and location.
Content Attribution
The morphs displayed here were originally prepared by Eric Martz in 2004 for the page Lac Repressor Binding to DNA, within ProteinExplorer.Org.
See Also
- Category: Lac repressor and Category: Lac Repressor, automatically-generated pages that list PDB codes for lac repressor models.
- Morphs where the morph of the lac repressor is used as an example.
References & Notes
- ↑ L'opéron: groupe de gènes à expression coordonée par un opérateur. [Operon: a group of genes with the expression coordinated by an operator.] C R Hebd Seances Acad Sci., 250:1727-9, 1960. PubMed 14406329
- ↑ The lac repressor. Lewis, M. C R Biol. 328:521-48, 2005. PubMed 15950160
- ↑ This domain coloring scheme is adapted from Fig. 6 in the review by Lewis (C. R. Biol. 328:521, 2005). Domains are 1-45, 46-62, (63-162,291-320), (163-290,321-332), 330-339, and 340-357.
- ↑ Conservation results for 1lbg are from the precalculated ConSurf Database, using 103 sequences from Swiss-Prot with an average pairwise distance of 2.4.
- ↑ Conservation results for 1lbi are from the ConSurf Server, using 100 sequences from Uniprot with an average pairwise distance of 1.3.
- ↑ 6.0 6.1 For these scenes, the 20-model PDB files for 1osl and 1l1m were reduced in size, to avoid exceeding the java memory available to the Jmol applet. All atoms except amino acid alpha carbons and DNA phosphorus atoms were removed using the free program alphac.exe from PDBTools. Secondary structure HELIX records from the original PDB file header were retained. The results are Image:1osl ca.pdb and Image:1l1m ca.pdb.
- ↑ Powerpoint is a registered trademark for a software package licensed by Microsoft Corp..