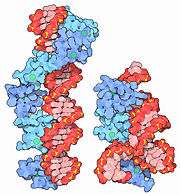
Cartoon representation for Zinc Finger
1zaa with Zn atoms (green)
Overview
Few classes of transcription factors are as significant as the zinc fingers. While there are zinc-containing subregions in other proteins, the distinguishing feature of a zinc finger is the spontaneous folding process which is facilitated by a . Since the zinc fingers are so small, they cannot rely on the collective strength of many hydrogen bonds or hydrophobic interactions to drive their folding. They are highly specific to their sequence and yet are able to target sequences common to multiple loci for regulation of a common function that may be influenced by many genes. They can also serve as a carrier for another domain which may bind covalently to the DNA, effecting permanent shutdown of a gene’s expression. Attesting to their importance in the human genome, a family of over 700 human proteins contain a zinc finger domain, a number exceeded only the immunoglobins, and then only slightly.
Basic Structural Profile of a Zinc Finger
The simplest group of zinc fingers, referred as C2H2 zinc fingers, consists of . The name C2H2, or Cis2His2, gives a nod to the residues involved in coordination of the zinc ion. The turn between the two β-pleated sheets forms a hydrophobic pocket near where the zinc ion is bound. Typically, the hydrophobic pocket is formed as a result of interactions between and leucines in close proximity. While both the coordination of the zinc ligand and the presence of the hydrophobic pocket stabilize the small zinc finger domain, the zinc ligand is responsible for a majority of the stability imparted to the motif. Because cells contain a highly reducing environment, sulfide bridges are unable to stabilize small protein domains. With a single oxidation state and the ability to accommodate both nitrogen and sulfur, Zinc is an ideal stabilizer. Due to the structural stability imparted to the motif by the zinc ion, zinc fingers are considerably smaller than most other proteins, usually ranging between 25 and 30 amino acids in length.
As a corollary of their small size, they are extremely agile and mobile, and so are the genes that encode them. Thus, they are easily able to bind to DNA, among other substrates, in part due to interactions of with the negatively charged phosphate backbone. By slipping into the major groove of DNA, they are able to use their to check for proper base identity. This allows the zinc fingers to bind to sequences normally unavailable to other, larger DNA-binding structural motifs.
As a family, the structure of the zinc fingers is as polymorphous as it is unique. Some are coordinated primarily or exclusively by cysteine, and many form much more complex structures than the β-hairpin of Cis2His2 types.
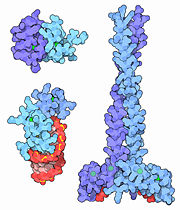
Example of a zinc finger: Gal4
is a positive regulator of galactose-induced genes, and is used in yeast two hybrid systems. The N terminal fragment of Gal4 binds as a dimer to a symmetrical 17-base-pair sequence. Each subunit folds into 3 distinct modules: A compact, , and an extended (41-49), and an alpha-helical element (50-64). A small, Zn(2+)-containing domain recognizes a conserved triplet at each end of the site through with the major groove. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4.
Biological Role and Regulation
Given the prevalence of the zinc fingers in nature, it is no surprise to learn that they are key players in many biological processes. Gli-1, a zinc-finger transcription factor involved in embryonic development, is also implicated in carcinogenesis when it is over-expressed in dividing cells. Likewise, Gli-3 is another zinc-finger transcription factor whose shortened repressor form has roles in modulating regulation of genes controlling apoptosis, and consequently has a dramatic effect on development of the limb bud and the digit formation that follows. In part, the regulation of the zinc fingers is kept in check by other repressors or activators, as well as various types of chromatin remodeling (e.g. acetylation, methylation) which definitively blocks transcription of their targets.
Clinical Applications
The advantages of using ZFPs in a clinical setting are numerous, the least of which is not their ability to bind DNA. As a result, the ZFP must bind two only two copies of its target, as opposed to therapeutics directed at mRNA, for example, which have to are in direct quantitative competition with their target. Because of this unique advantage as well as their modularity and ability to work in clusters, the ZFPs are currently the subject of extensive research for use in genetic therapy. Their versatility and modularity among targets can be explained as a function of their α-helical sidechains, which they use to interact electrostatically with multiple sequences. Because they can withstand multiple mutations without losing their functional structure, they make great candidates for specific gene-targeting applications. In theory, the zinc finger as a therapeutic agent could unilaterally control gene expression, given that a transcription factor had been synthesized which possessed the proper sequence identity for binding to the gene or the regulated protein.