Structural highlights
Function
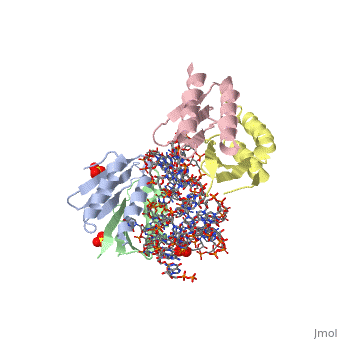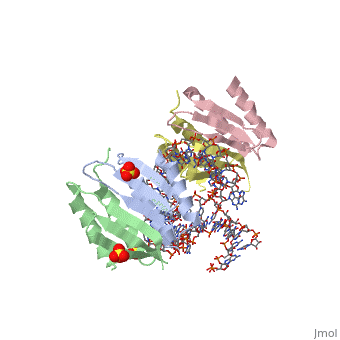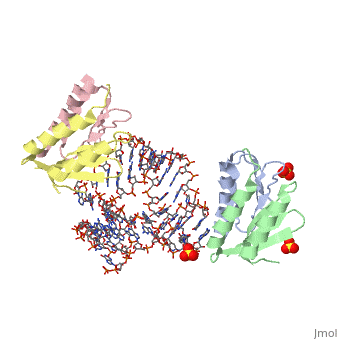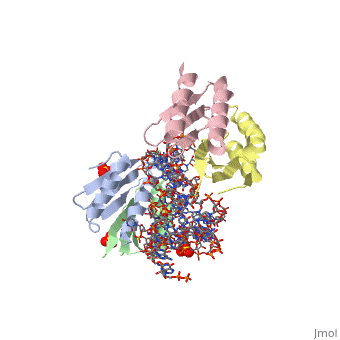
SRP09_HUMAN Signal-recognition-particle assembly has a crucial role in targeting secretory proteins to the rough endoplasmic reticulum membrane. SRP9 together with SRP14 and the Alu portion of the SRP RNA, constitutes the elongation arrest domain of SRP. The complex of SRP9 and SRP14 is required for SRP RNA binding.
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
The Alu domain of the mammalian signal recognition particle (SRP) comprises the heterodimer of proteins SRP9 and SRP14 bound to the 5' and 3' terminal sequences of SRP RNA. It retards the ribosomal elongation of signal-peptide-containing proteins before their engagement with the translocation machinery in the endoplasmic reticulum. Here we report two crystal structures of the heterodimer SRP9/14 bound either to the 5' domain or to a construct containing both 5' and 3' domains. We present a model of the complete Alu domain that is consistent with extensive biochemical data. SRP9/14 binds strongly to the conserved core of the 5' domain, which forms a U-turn connecting two helical stacks. Reversible docking of the more weakly bound 3' domain might be functionally important in the mechanism of translational regulation. The Alu domain structure is probably conserved in other cytoplasmic ribonucleoprotein particles and retroposition intermediates containing SRP9/14-bound RNAs transcribed from Alu repeats or related elements in genomic DNA.
Structure and assembly of the Alu domain of the mammalian signal recognition particle.,Weichenrieder O, Wild K, Strub K, Cusack S Nature. 2000 Nov 9;408(6809):167-73. PMID:11089964[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Weichenrieder O, Wild K, Strub K, Cusack S. Structure and assembly of the Alu domain of the mammalian signal recognition particle. Nature. 2000 Nov 9;408(6809):167-73. PMID:11089964 doi:10.1038/35041507