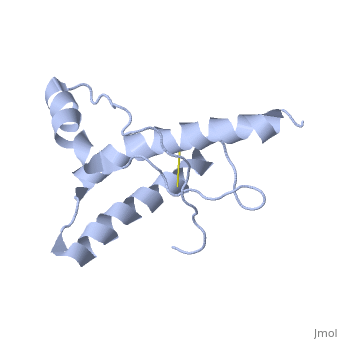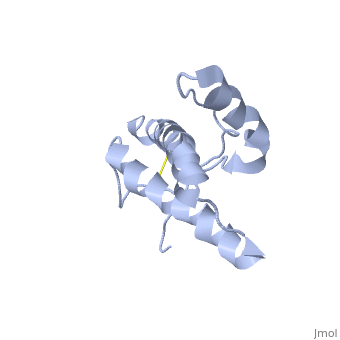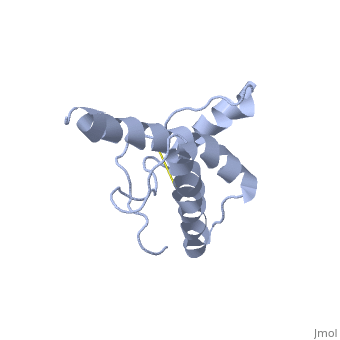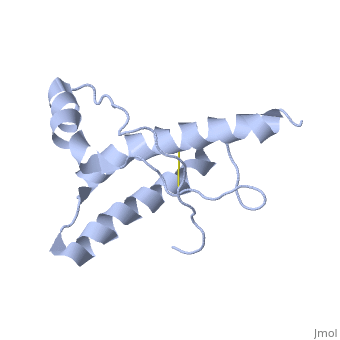
| Structural highlights
Disease
PRIO_HUMAN Note=PrP is found in high quantity in the brain of humans and animals infected with neurodegenerative diseases known as transmissible spongiform encephalopathies or prion diseases, like: Creutzfeldt-Jakob disease (CJD), fatal familial insomnia (FFI), Gerstmann-Straussler disease (GSD), Huntington disease-like type 1 (HDL1) and kuru in humans; scrapie in sheep and goat; bovine spongiform encephalopathy (BSE) in cattle; transmissible mink encephalopathy (TME); chronic wasting disease (CWD) of mule deer and elk; feline spongiform encephalopathy (FSE) in cats and exotic ungulate encephalopathy (EUE) in nyala and greater kudu. The prion diseases illustrate three manifestations of CNS degeneration: (1) infectious (2) sporadic and (3) dominantly inherited forms. TME, CWD, BSE, FSE, EUE are all thought to occur after consumption of prion-infected foodstuffs.[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] Defects in PRNP are the cause of Creutzfeldt-Jakob disease (CJD) [MIM:123400. CJD occurs primarily as a sporadic disorder (1 per million), while 10-15% are familial. Accidental transmission of CJD to humans appears to be iatrogenic (contaminated human growth hormone (HGH), corneal transplantation, electroencephalographic electrode implantation, etc.). Epidemiologic studies have failed to implicate the ingestion of infected annimal meat in the pathogenesis of CJD in human. The triad of microscopic features that characterize the prion diseases consists of (1) spongiform degeneration of neurons, (2) severe astrocytic gliosis that often appears to be out of proportion to the degree of nerve cell loss, and (3) amyloid plaque formation. CJD is characterized by progressive dementia and myoclonic seizures, affecting adults in mid-life. Some patients present sleep disorders, abnormalities of high cortical function, cerebellar and corticospinal disturbances. The disease ends in death after a 3-12 months illness.[11] [12] [13] [14] [15] [16] [17] [18] [19] [20] Defects in PRNP are the cause of fatal familial insomnia (FFI) [MIM:600072. FFI is an autosomal dominant disorder and is characterized by neuronal degeneration limited to selected thalamic nuclei and progressive insomnia.[21] [22] [23] Defects in PRNP are the cause of Gerstmann-Straussler disease (GSD) [MIM:137440. GSD is a heterogeneous disorder and was defined as a spinocerebellar ataxia with dementia and plaquelike deposits. GSD incidence is less than 2 per 100 million live births.[24] [25] [26] [27] [28] [29] [30] [31] [32] [33] [34] Defects in PRNP are the cause of Huntington disease-like type 1 (HDL1) [MIM:603218. HDL1 is an autosomal dominant, early onset neurodegenerative disorder with prominent psychiatric features.[35] Defects in PRNP are the cause of kuru (KURU) [MIM:245300. Kuru is transmitted during ritualistic cannibalism, among natives of the New Guinea highlands. Patients exhibit various movement disorders like cerebellar abnormalities, rigidity of the limbs, and clonus. Emotional lability is present, and dementia is conspicuously absent. Death usually occurs from 3 to 12 month after onset.[36] Defects in PRNP are the cause of spongiform encephalopathy with neuropsychiatric features (SENF) [MIM:606688; an autosomal dominant presenile dementia with a rapidly progressive and protracted clinical course. The dementia was characterized clinically by frontotemporal features, including early personality changes. Some patients had memory loss, several showed aggressiveness, hyperorality and verbal stereotypy, others had parkinsonian symptoms.[37]
Function
PRIO_HUMAN May play a role in neuronal development and synaptic plasticity. May be required for neuronal myelin sheath maintenance. May play a role in iron uptake and iron homeostasis. Soluble oligomers are toxic to cultured neuroblastoma cells and induce apoptosis (in vitro). Association with GPC1 (via its heparan sulfate chains) targets PRNP to lipid rafts. Also provides Cu(2+) or ZN(2+) for the ascorbate-mediated GPC1 deaminase degradation of its heparan sulfate side chains (By similarity).[38] [39] [40]
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
The NMR structure of the globular domain of the human prion protein (hPrP) with residues 121-230 at pH 7.0 shows the same global fold as the previously published structure determined at pH 4.5. It contains three alpha-helices, comprising residues 144-156, 174-194, and 200-228, and a short anti-parallel beta-sheet, comprising residues 128-131 and 161-164. There are slight, strictly localized, conformational changes at neutral pH when compared with acidic solution conditions: helix alpha1 is elongated at the C-terminal end with residues 153-156 forming a 310-helix, and the population of helical structure in the C-terminal two turns of helix alpha 2 is increased. The protonation of His155 and His187 presumably contributes to these structural changes. Thermal unfolding monitored by far UV CD indicates that hPrP-(121-230) is significantly more stable at neutral pH. Measurements of amide proton protection factors map local differences in protein stability within residues 154-157 at the C-terminal end of helix alpha 1 and residues 161-164 of beta-strand 2. These two segments appear to form a separate domain that at acidic pH has a larger tendency to unfold than the overall protein structure. This domain could provide a "starting point" for pH-induced unfolding and thus may be implicated in endosomic PrPC to PrPSc conformational transition resulting in transmissible spongiform encephalopathies.
Influence of pH on NMR structure and stability of the human prion protein globular domain.,Calzolai L, Zahn R J Biol Chem. 2003 Sep 12;278(37):35592-6. Epub 2003 Jun 25. PMID:12826672[41]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Taylor DR, Whitehouse IJ, Hooper NM. Glypican-1 mediates both prion protein lipid raft association and disease isoform formation. PLoS Pathog. 2009 Nov;5(11):e1000666. doi: 10.1371/journal.ppat.1000666. Epub, 2009 Nov 20. PMID:19936054 doi:10.1371/journal.ppat.1000666
- ↑ Goldfarb LG, Haltia M, Brown P, Nieto A, Kovanen J, McCombie WR, Trapp S, Gajdusek DC. New mutation in scrapie amyloid precursor gene (at codon 178) in Finnish Creutzfeldt-Jakob kindred. Lancet. 1991 Feb 16;337(8738):425. PMID:1671440
- ↑ Goldfarb LG, Mitrova E, Brown P, Toh BK, Gajdusek DC. Mutation in codon 200 of scrapie amyloid protein gene in two clusters of Creutzfeldt-Jakob disease in Slovakia. Lancet. 1990 Aug 25;336(8713):514-5. PMID:1975028
- ↑ Kitamoto T, Ohta M, Doh-ura K, Hitoshi S, Terao Y, Tateishi J. Novel missense variants of prion protein in Creutzfeldt-Jakob disease or Gerstmann-Straussler syndrome. Biochem Biophys Res Commun. 1993 Mar 15;191(2):709-14. PMID:8461023
- ↑ Pocchiari M, Salvatore M, Cutruzzola F, Genuardi M, Allocatelli CT, Masullo C, Macchi G, Alema G, Galgani S, Xi YG, et al.. A new point mutation of the prion protein gene in Creutzfeldt-Jakob disease. Ann Neurol. 1993 Dec;34(6):802-7. PMID:7902693 doi:http://dx.doi.org/10.1002/ana.410340608
- ↑ Inoue I, Kitamoto T, Doh-ura K, Shii H, Goto I, Tateishi J. Japanese family with Creutzfeldt-Jakob disease with codon 200 point mutation of the prion protein gene. Neurology. 1994 Feb;44(2):299-301. PMID:7906019
- ↑ Gabizon R, Rosenman H, Meiner Z, Kahana I, Kahana E, Shugart Y, Ott J, Prusiner SB. Mutation in codon 200 and polymorphism in codon 129 of the prion protein gene in Libyan Jews with Creutzfeldt-Jakob disease. Philos Trans R Soc Lond B Biol Sci. 1994 Mar 29;343(1306):385-90. PMID:7913755 doi:http://dx.doi.org/10.1098/rstb.1994.0033
- ↑ Mastrianni JA, Iannicola C, Myers RM, DeArmond S, Prusiner SB. Mutation of the prion protein gene at codon 208 in familial Creutzfeldt-Jakob disease. Neurology. 1996 Nov;47(5):1305-12. PMID:8909447
- ↑ Nitrini R, Rosemberg S, Passos-Bueno MR, da Silva LS, Iughetti P, Papadopoulos M, Carrilho PM, Caramelli P, Albrecht S, Zatz M, LeBlanc A. Familial spongiform encephalopathy associated with a novel prion protein gene mutation. Ann Neurol. 1997 Aug;42(2):138-46. PMID:9266722 doi:10.1002/ana.410420203
- ↑ Peoc'h K, Manivet P, Beaudry P, Attane F, Besson G, Hannequin D, Delasnerie-Laupretre N, Laplanche JL. Identification of three novel mutations (E196K, V203I, E211Q) in the prion protein gene (PRNP) in inherited prion diseases with Creutzfeldt-Jakob disease phenotype. Hum Mutat. 2000 May;15(5):482. PMID:10790216 doi:<482::AID-HUMU16>3.0.CO;2-1 10.1002/(SICI)1098-1004(200005)15:5<482::AID-HUMU16>3.0.CO;2-1
- ↑ Taylor DR, Whitehouse IJ, Hooper NM. Glypican-1 mediates both prion protein lipid raft association and disease isoform formation. PLoS Pathog. 2009 Nov;5(11):e1000666. doi: 10.1371/journal.ppat.1000666. Epub, 2009 Nov 20. PMID:19936054 doi:10.1371/journal.ppat.1000666
- ↑ Goldfarb LG, Haltia M, Brown P, Nieto A, Kovanen J, McCombie WR, Trapp S, Gajdusek DC. New mutation in scrapie amyloid precursor gene (at codon 178) in Finnish Creutzfeldt-Jakob kindred. Lancet. 1991 Feb 16;337(8738):425. PMID:1671440
- ↑ Goldfarb LG, Mitrova E, Brown P, Toh BK, Gajdusek DC. Mutation in codon 200 of scrapie amyloid protein gene in two clusters of Creutzfeldt-Jakob disease in Slovakia. Lancet. 1990 Aug 25;336(8713):514-5. PMID:1975028
- ↑ Kitamoto T, Ohta M, Doh-ura K, Hitoshi S, Terao Y, Tateishi J. Novel missense variants of prion protein in Creutzfeldt-Jakob disease or Gerstmann-Straussler syndrome. Biochem Biophys Res Commun. 1993 Mar 15;191(2):709-14. PMID:8461023
- ↑ Pocchiari M, Salvatore M, Cutruzzola F, Genuardi M, Allocatelli CT, Masullo C, Macchi G, Alema G, Galgani S, Xi YG, et al.. A new point mutation of the prion protein gene in Creutzfeldt-Jakob disease. Ann Neurol. 1993 Dec;34(6):802-7. PMID:7902693 doi:http://dx.doi.org/10.1002/ana.410340608
- ↑ Inoue I, Kitamoto T, Doh-ura K, Shii H, Goto I, Tateishi J. Japanese family with Creutzfeldt-Jakob disease with codon 200 point mutation of the prion protein gene. Neurology. 1994 Feb;44(2):299-301. PMID:7906019
- ↑ Gabizon R, Rosenman H, Meiner Z, Kahana I, Kahana E, Shugart Y, Ott J, Prusiner SB. Mutation in codon 200 and polymorphism in codon 129 of the prion protein gene in Libyan Jews with Creutzfeldt-Jakob disease. Philos Trans R Soc Lond B Biol Sci. 1994 Mar 29;343(1306):385-90. PMID:7913755 doi:http://dx.doi.org/10.1098/rstb.1994.0033
- ↑ Mastrianni JA, Iannicola C, Myers RM, DeArmond S, Prusiner SB. Mutation of the prion protein gene at codon 208 in familial Creutzfeldt-Jakob disease. Neurology. 1996 Nov;47(5):1305-12. PMID:8909447
- ↑ Nitrini R, Rosemberg S, Passos-Bueno MR, da Silva LS, Iughetti P, Papadopoulos M, Carrilho PM, Caramelli P, Albrecht S, Zatz M, LeBlanc A. Familial spongiform encephalopathy associated with a novel prion protein gene mutation. Ann Neurol. 1997 Aug;42(2):138-46. PMID:9266722 doi:10.1002/ana.410420203
- ↑ Peoc'h K, Manivet P, Beaudry P, Attane F, Besson G, Hannequin D, Delasnerie-Laupretre N, Laplanche JL. Identification of three novel mutations (E196K, V203I, E211Q) in the prion protein gene (PRNP) in inherited prion diseases with Creutzfeldt-Jakob disease phenotype. Hum Mutat. 2000 May;15(5):482. PMID:10790216 doi:<482::AID-HUMU16>3.0.CO;2-1 10.1002/(SICI)1098-1004(200005)15:5<482::AID-HUMU16>3.0.CO;2-1
- ↑ Taylor DR, Whitehouse IJ, Hooper NM. Glypican-1 mediates both prion protein lipid raft association and disease isoform formation. PLoS Pathog. 2009 Nov;5(11):e1000666. doi: 10.1371/journal.ppat.1000666. Epub, 2009 Nov 20. PMID:19936054 doi:10.1371/journal.ppat.1000666
- ↑ Lee S, Antony L, Hartmann R, Knaus KJ, Surewicz K, Surewicz WK, Yee VC. Conformational diversity in prion protein variants influences intermolecular beta-sheet formation. EMBO J. 2010 Jan 6;29(1):251-62. Epub 2009 Nov 19. PMID:19927125 doi:10.1038/emboj.2009.333
- ↑ Medori R, Montagna P, Tritschler HJ, LeBlanc A, Cortelli P, Tinuper P, Lugaresi E, Gambetti P. Fatal familial insomnia: a second kindred with mutation of prion protein gene at codon 178. Neurology. 1992 Mar;42(3 Pt 1):669-70. PMID:1347910
- ↑ Taylor DR, Whitehouse IJ, Hooper NM. Glypican-1 mediates both prion protein lipid raft association and disease isoform formation. PLoS Pathog. 2009 Nov;5(11):e1000666. doi: 10.1371/journal.ppat.1000666. Epub, 2009 Nov 20. PMID:19936054 doi:10.1371/journal.ppat.1000666
- ↑ Lee S, Antony L, Hartmann R, Knaus KJ, Surewicz K, Surewicz WK, Yee VC. Conformational diversity in prion protein variants influences intermolecular beta-sheet formation. EMBO J. 2010 Jan 6;29(1):251-62. Epub 2009 Nov 19. PMID:19927125 doi:10.1038/emboj.2009.333
- ↑ Cervenakova L, Buetefisch C, Lee HS, Taller I, Stone G, Gibbs CJ Jr, Brown P, Hallett M, Goldfarb LG. Novel PRNP sequence variant associated with familial encephalopathy. Am J Med Genet. 1999 Dec 15;88(6):653-6. PMID:10581485
- ↑ Hsiao K, Baker HF, Crow TJ, Poulter M, Owen F, Terwilliger JD, Westaway D, Ott J, Prusiner SB. Linkage of a prion protein missense variant to Gerstmann-Straussler syndrome. Nature. 1989 Mar 23;338(6213):342-5. PMID:2564168 doi:http://dx.doi.org/10.1038/338342a0
- ↑ Hsiao K, Dlouhy SR, Farlow MR, Cass C, Da Costa M, Conneally PM, Hodes ME, Ghetti B, Prusiner SB. Mutant prion proteins in Gerstmann-Straussler-Scheinker disease with neurofibrillary tangles. Nat Genet. 1992 Apr;1(1):68-71. PMID:1363810 doi:http://dx.doi.org/10.1038/ng0492-68
- ↑ Yamada M, Itoh Y, Fujigasaki H, Naruse S, Kaneko K, Kitamoto T, Tateishi J, Otomo E, Hayakawa M, Tanaka J, et al.. A missense mutation at codon 105 with codon 129 polymorphism of the prion protein gene in a new variant of Gerstmann-Straussler-Scheinker disease. Neurology. 1993 Dec;43(12):2723-4. PMID:7902972
- ↑ Itoh Y, Yamada M, Hayakawa M, Shozawa T, Tanaka J, Matsushita M, Kitamoto T, Tateishi J, Otomo E. A variant of Gerstmann-Straussler-Scheinker disease carrying codon 105 mutation with codon 129 polymorphism of the prion protein gene: a clinicopathological study. J Neurol Sci. 1994 Dec 1;127(1):77-86. PMID:7699395
- ↑ Young K, Jones CK, Piccardo P, Lazzarini A, Golbe LI, Zimmerman TR Jr, Dickson DW, McLachlan DC, St George-Hyslop P, Lennox A, et al.. Gerstmann-Straussler-Scheinker disease with mutation at codon 102 and methionine at codon 129 of PRNP in previously unreported patients. Neurology. 1995 Jun;45(6):1127-34. PMID:7783876
- ↑ Barbanti P, Fabbrini G, Salvatore M, Petraroli R, Cardone F, Maras B, Equestre M, Macchi G, Lenzi GL, Pocchiari M. Polymorphism at codon 129 or codon 219 of PRNP and clinical heterogeneity in a previously unreported family with Gerstmann-Straussler-Scheinker disease (PrP-P102L mutation). Neurology. 1996 Sep;47(3):734-41. PMID:8797472
- ↑ Piccardo P, Dlouhy SR, Lievens PM, Young K, Bird TD, Nochlin D, Dickson DW, Vinters HV, Zimmerman TR, Mackenzie IR, Kish SJ, Ang LC, De Carli C, Pocchiari M, Brown P, Gibbs CJ Jr, Gajdusek DC, Bugiani O, Ironside J, Tagliavini F, Ghetti B. Phenotypic variability of Gerstmann-Straussler-Scheinker disease is associated with prion protein heterogeneity. J Neuropathol Exp Neurol. 1998 Oct;57(10):979-88. PMID:9786248
- ↑ Panegyres PK, Toufexis K, Kakulas BA, Cernevakova L, Brown P, Ghetti B, Piccardo P, Dlouhy SR. A new PRNP mutation (G131V) associated with Gerstmann-Straussler-Scheinker disease. Arch Neurol. 2001 Nov;58(11):1899-902. PMID:11709001
- ↑ Taylor DR, Whitehouse IJ, Hooper NM. Glypican-1 mediates both prion protein lipid raft association and disease isoform formation. PLoS Pathog. 2009 Nov;5(11):e1000666. doi: 10.1371/journal.ppat.1000666. Epub, 2009 Nov 20. PMID:19936054 doi:10.1371/journal.ppat.1000666
- ↑ Taylor DR, Whitehouse IJ, Hooper NM. Glypican-1 mediates both prion protein lipid raft association and disease isoform formation. PLoS Pathog. 2009 Nov;5(11):e1000666. doi: 10.1371/journal.ppat.1000666. Epub, 2009 Nov 20. PMID:19936054 doi:10.1371/journal.ppat.1000666
- ↑ Taylor DR, Whitehouse IJ, Hooper NM. Glypican-1 mediates both prion protein lipid raft association and disease isoform formation. PLoS Pathog. 2009 Nov;5(11):e1000666. doi: 10.1371/journal.ppat.1000666. Epub, 2009 Nov 20. PMID:19936054 doi:10.1371/journal.ppat.1000666
- ↑ Mani K, Cheng F, Havsmark B, Jonsson M, Belting M, Fransson LA. Prion, amyloid beta-derived Cu(II) ions, or free Zn(II) ions support S-nitroso-dependent autocleavage of glypican-1 heparan sulfate. J Biol Chem. 2003 Oct 3;278(40):38956-65. Epub 2003 May 5. PMID:12732622 doi:10.1074/jbc.M300394200
- ↑ Taylor DR, Whitehouse IJ, Hooper NM. Glypican-1 mediates both prion protein lipid raft association and disease isoform formation. PLoS Pathog. 2009 Nov;5(11):e1000666. doi: 10.1371/journal.ppat.1000666. Epub, 2009 Nov 20. PMID:19936054 doi:10.1371/journal.ppat.1000666
- ↑ Wu D, Zhang W, Luo Q, Luo K, Huang L, Wang W, Huang T, Chen R, Lin Y, Pang D, Xiao G. Copper (II) promotes the formation of soluble neurotoxic PrP oligomers in acidic environment. J Cell Biochem. 2010 Oct 15;111(3):627-33. doi: 10.1002/jcb.22743. PMID:20564047 doi:10.1002/jcb.22743
- ↑ Calzolai L, Zahn R. Influence of pH on NMR structure and stability of the human prion protein globular domain. J Biol Chem. 2003 Sep 12;278(37):35592-6. Epub 2003 Jun 25. PMID:12826672 doi:10.1074/jbc.M303005200
|