Structural highlights
Function
PBP_BOMMO This major soluble protein in olfactory sensilla of male moths serves to solubilize the extremely hydrophobic pheromone molecules such as bombykol and to transport pheromone through the aqueous lymph to receptors located on olfactory cilia.
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
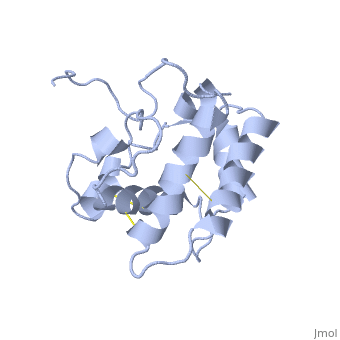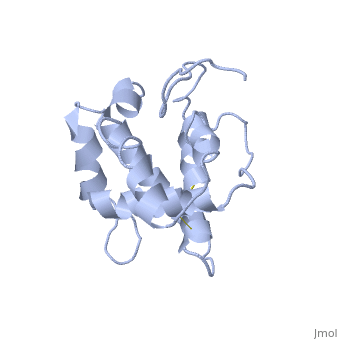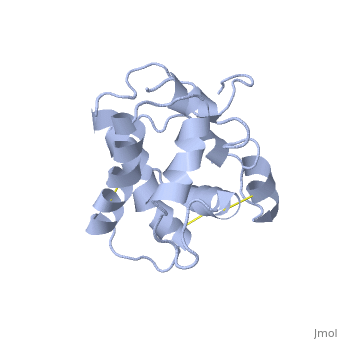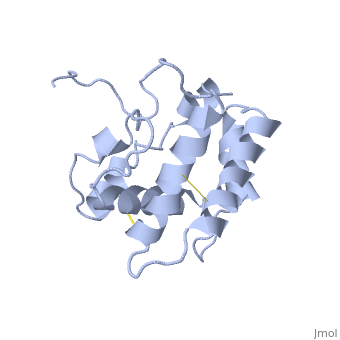
The nuclear magnetic resonance structure of the unliganded pheromone-binding protein (PBP) from Bombyx mori at pH above 6.5, BmPBP(B), consists of seven helices with residues 3-8, 16-22, 29-32, 46-59, 70-79, 84-100, and 107-124, and contains the three disulfide bridges 19-54, 50-108, and 97-117. This polypeptide fold encloses a large hydrophobic cavity, with a sufficient volume to accommodate the natural ligand bombykol. The polypeptide folds in free BmPBP(B) and in crystals of a BmPBP-bombykol complex are nearly identical, indicating that the B-form of BmPBP in solution represents the active conformation for ligand binding.
NMR structure of the unliganded Bombyx mori pheromone-binding protein at physiological pH.,Lee D, Damberger FF, Peng G, Horst R, Guntert P, Nikonova L, Leal WS, Wuthrich K FEBS Lett. 2002 Nov 6;531(2):314-8. PMID:12417333[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Lee D, Damberger FF, Peng G, Horst R, Guntert P, Nikonova L, Leal WS, Wuthrich K. NMR structure of the unliganded Bombyx mori pheromone-binding protein at physiological pH. FEBS Lett. 2002 Nov 6;531(2):314-8. PMID:12417333