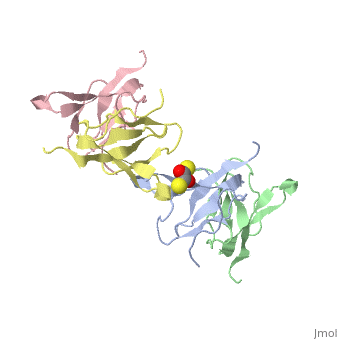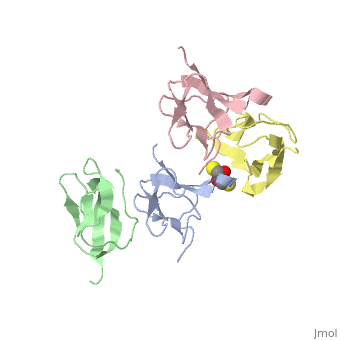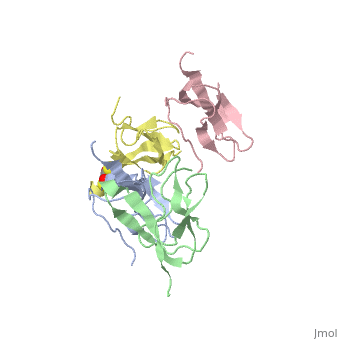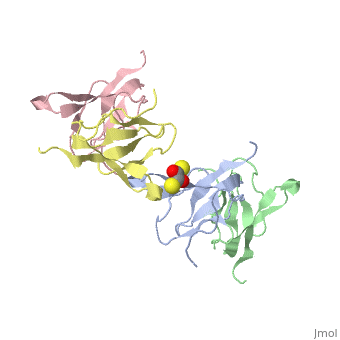Structural highlights
Function
RL27_THET8 Found on the solvent side of the large subunit.[HAMAP-Rule:MF_00539]
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
Ribosomal protein L27 is located near the peptidyltransferase center at the interface of ribosomal subunits, and is important for ribosomal assembly and function. We report the crystal structure of ribosomal protein L27 from Thermus thermophilus HB8, which was determined by the multiwavelength anomalous dispersion method and refined to an R-factor of 19.7% (R(free) = 23.6%) at 2.8 A resolution. The overall fold is an all beta-sheet hybrid. It consists of two sets of four-stranded beta-sheets formed around a well-defined hydrophobic core, with a highly positive charge on the protein surface. The structure of ribosomal protein L27 from T. thermophilus HB8 in the RNA-free form is investigated, and its functional roles in the ribosomal subunit are discussed.
Crystal structure of ribosomal protein L27 from Thermus thermophilus HB8.,Wang H, Takemoto CH, Murayama K, Sakai H, Tatsuguchi A, Terada T, Shirouzu M, Kuramitsu S, Yokoyama S Protein Sci. 2004 Oct;13(10):2806-10. Epub 2004 Aug 31. PMID:15340170[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Wang H, Takemoto CH, Murayama K, Sakai H, Tatsuguchi A, Terada T, Shirouzu M, Kuramitsu S, Yokoyama S. Crystal structure of ribosomal protein L27 from Thermus thermophilus HB8. Protein Sci. 2004 Oct;13(10):2806-10. Epub 2004 Aug 31. PMID:15340170 doi:10.1110/ps.04864904