Structural highlights
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
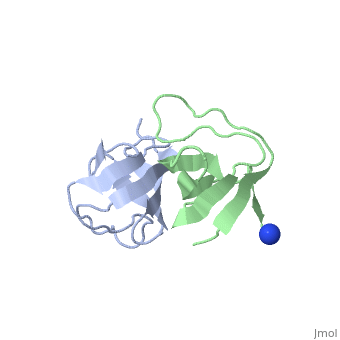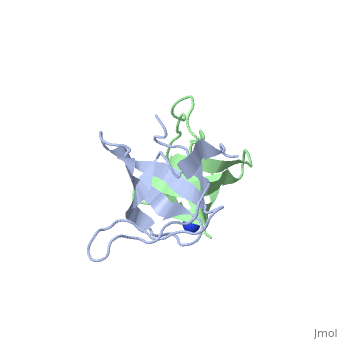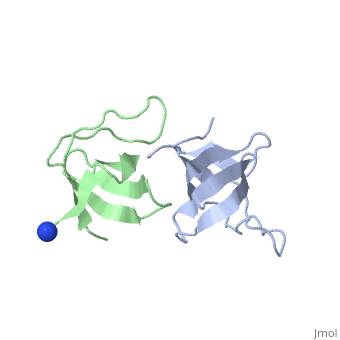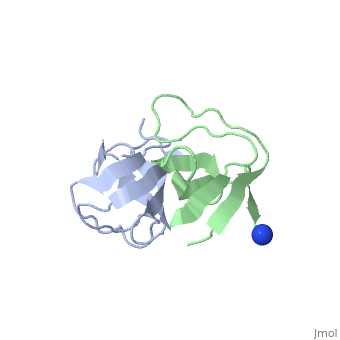
X-ray structures of two crystal forms of the Src homology 3 domain (SH3) of the Ras GTPase activating protein (RasGAP) were determined at 1.5 and 1.8A resolution. The overall structure comprises a single domain with two tightly packed beta-sheets linked by a short helical segment. An important motif for peptide binding in other SH3 domains is not conserved in RasGAP. The RasGAP SH3 domain forms dimers in the crystal structures, which may provide new functional insight. The dimer interface involves residues also present in a peptide previously identified as an apoptotic sensitizer of tumor cells.
High resolution crystal structures of the p120 RasGAP SH3 domain.,Ross B, Kristensen O, Favre D, Walicki J, Kastrup JS, Widmann C, Gajhede M Biochem Biophys Res Commun. 2007 Feb 9;353(2):463-8. Epub 2006 Dec 18. PMID:17188236[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Ross B, Kristensen O, Favre D, Walicki J, Kastrup JS, Widmann C, Gajhede M. High resolution crystal structures of the p120 RasGAP SH3 domain. Biochem Biophys Res Commun. 2007 Feb 9;353(2):463-8. Epub 2006 Dec 18. PMID:17188236 doi:http://dx.doi.org/10.1016/j.bbrc.2006.12.044