2kdg
From Proteopedia
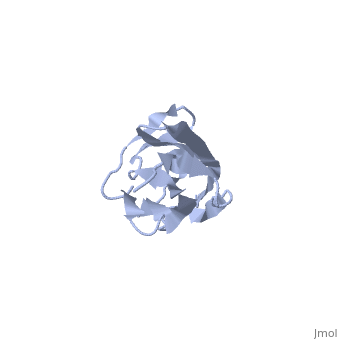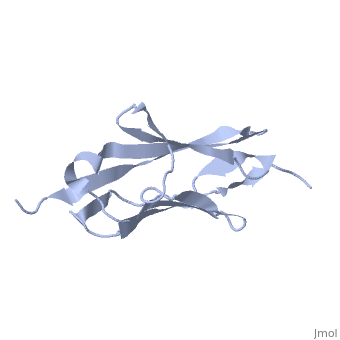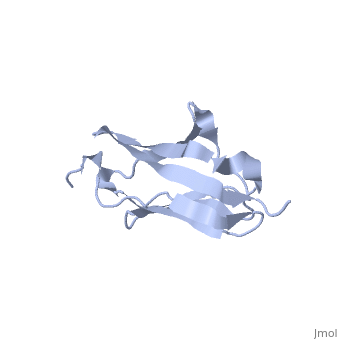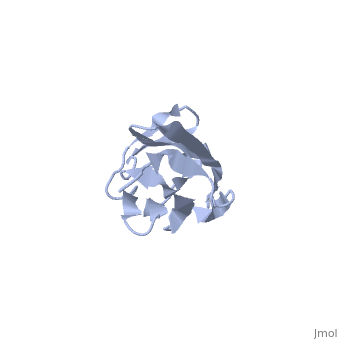
Solution Structure of the 1st Ig domain of Myotilin
Structural highlights
DiseaseMYOTI_HUMAN Defects in MYOT are the cause of limb-girdle muscular dystrophy type 1A (LGMD1A) [MIM:159000. LGMD1A is an autosomal dominant degenerative myopathy with onset within a mean age of 28 years. LGMD1A is characterized by progressive skeletal muscle weakness of the hip and shoulder girdles, later progressing to include distal weakness, as well as a distinctive dysarthric pattern of speech. Affected muscle exhibits disorganization and streaming of the Z-line.[1] [2] Defects in MYOT are the cause of myopathy myofibrillar type 3 (MFM3) [MIM:609200. A neuromuscular disorder characterized by progressive skeletal muscle weakness greater distally than proximally, tight heel cords, hyporeflexia, cardiomyopathy and peripheral neuropathy in some patients. Affected muscle exhibits disorganization and streaming of the Z-line, presence of large hyaline structures, excessive accumulation of myotilin and other ectopically expressed proteins and prominent congophilic deposits.[3] Defects in MYOT are the cause of spheroid body myopathy (SBM) [MIM:182920. SBM is an autosomal dominant form of myofibrillar myopathy (MFM), characterized by slowly progressing proximal muscle weakness and dysarthric nasal speech. There is no evidence of cardiomyopathy. Muscle biopsy shows spheroid bodies within the type I muscle fibers.[4] FunctionMYOTI_HUMAN Component of a complex of multiple actin cross-linking proteins. Involved in the control of myofibril assembly and stability at the Z lines in muscle cells.[5] Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedMyotilin is a 57 kDa actin-binding and -bundling protein that consists of a unique serine-rich amino-terminus, two Ig-domains and a short carboxy-terminus with a PDZ-binding motif. Myotilin localizes in sarcomeric Z-discs, where it interacts with several sarcomeric proteins. Point mutations in myotilin cause muscle disorders morphologically highlighted by sarcomeric disarray and aggregation. The actin-binding and dimerization propensity of myotilin has been mapped to the Ig-domains. Here we present high-resolution structure of the first Ig-domain of myotilin (MyoIg1) determined with solution state NMR spectroscopy. Nearly complete chemical shift assignments of MyoIg1 were achieved despite several missing backbone 1H-15N-HSQC signals. The structure derived from distance and dihedral angle restraints using torsion angle dynamics was further refined using molecular dynamics. The structure of MyoIg1 exhibits I-type Ig-fold. The absence of several backbone 1H-15N-HSQC signals can be explained by conformational exchange taking place at the hydrophobic core of the protein. Solution structure of the first immunoglobulin domain of human myotilin.,Heikkinen O, Permi P, Koskela H, Carpen O, Ylanne J, Kilpelainen I J Biomol NMR. 2009 Jun;44(2):107-12. Epub 2009 May 6. PMID:19418025[6] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
| ||||||||||||||||||