2km6
From Proteopedia
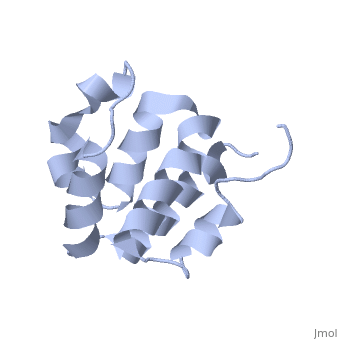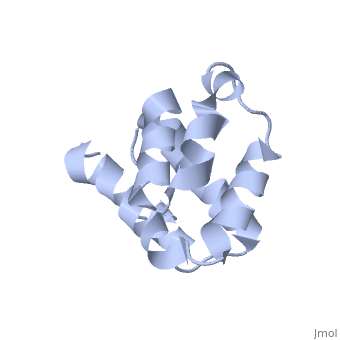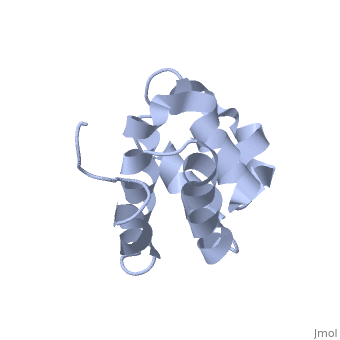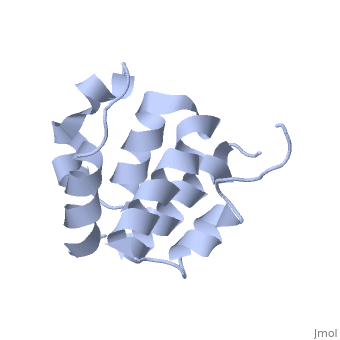
NMR structure of the NLRP7 Pyrin domain
Structural highlights
DiseaseNALP7_HUMAN Partial hydatidiform mole;Complete hydatidiform mole. The disease is caused by mutations affecting the gene represented in this entry.[1] [2] FunctionNALP7_HUMAN Inhibits CASP1/caspase-1-dependent IL1B secretion.[3] Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe innate immune system provides an initial line of defense against infection. Nucleotide-binding domain- and leucine-rich repeat-containing protein (NLR or (NOD-like)) receptors play a critical role in the innate immune response by surveying the cytoplasm for traces of intracellular invaders and endogenous stress signals. NLRs themselves are multi-domain proteins. Their N-terminal effector domains (typically a pyrin or caspase activation and recruitment domain) are responsible for driving downstream signaling and initiating the formation of inflammasomes, multi-component complexes necessary for cytokine activation. However, the currently available structures of NLR effector domains have not yet revealed the mechanism of their differential modes of interaction. Here, we report the structure and dynamics of the N-terminal pyrin domain of NLRP7 (NLRP7 PYD) obtained by NMR spectroscopy. The NLRP7 PYD adopts a six-alpha-helix bundle death domain fold. A comparison of conformational and dynamics features of the NLRP7 PYD with other PYDs showed distinct differences for helix alpha3 and loop alpha2-alpha3, which, in NLRP7, is stabilized by a strong hydrophobic cluster. Moreover, the NLRP7 and NLRP1 PYDs have different electrostatic surfaces. This is significant, because death domain signaling is driven by electrostatic contacts and stabilized by hydrophobic interactions. Thus, these results provide new insights into NLRP signaling and provide a first molecular understanding of inflammasome formation. Three-dimensional structure of the NLRP7 pyrin domain: insight into pyrin-pyrin-mediated effector domain signaling in innate immunity.,Pinheiro AS, Proell M, Eibl C, Page R, Schwarzenbacher R, Peti W J Biol Chem. 2010 Aug 27;285(35):27402-10. Epub 2010 Jun 11. PMID:20547486[4] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
| ||||||||||||||||||