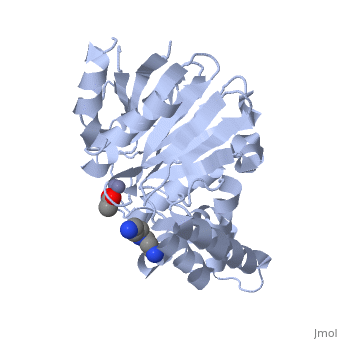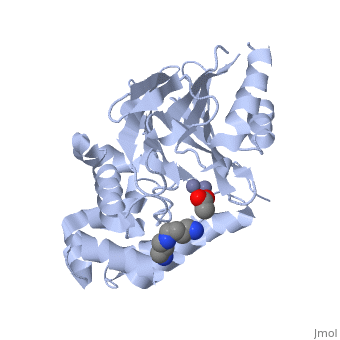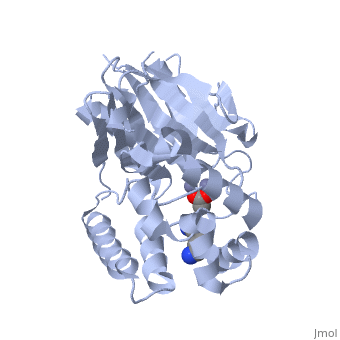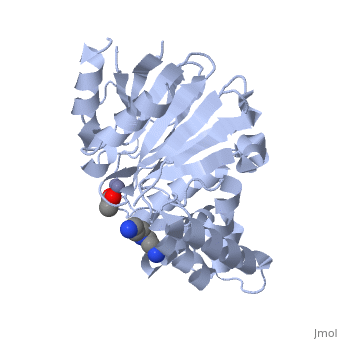
Structural highlights
Function
Q2PYN0_LEIIN
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
The glyoxalase pathway catalyzes the formation of d-lactate from methylglyoxal, a toxic byproduct of glycolysis. In trypanosomatids, trypanothione replaces glutathione in this pathway, making it a potential drug target, since its selective inhibition might increase methylglyoxal concentration in the parasites. Two glyoxalase II structures were solved. One with a bound spermidine molecule (1.8 A) and the other with d-lactate at the active site (1.9 A). The second structure was obtained by crystal soaking with the enzyme substrate (S)-d-lactoyltrypanothione. The overall structure of Leishmania infantum glyoxalase II is very similar to its human counterpart, with important differences at the substrate binding site. The crystal structure of L. infantum glyoxalase II is the first structure of this enzyme from trypanosomatids. The differential specificity of glyoxalase II toward glutathione and trypanothione moieties was revealed by differential substrate binding. Evolutionary analysis shows that trypanosomatid glyoxalases II diverged early from eukaryotic enzymes, being unrelated to prokaryotic proteins.
Catalysis and structural properties of Leishmania infantum glyoxalase II: trypanothione specificity and phylogeny.,Silva MS, Barata L, Ferreira AE, Romao S, Tomas AM, Freire AP, Cordeiro C Biochemistry. 2008 Jan 8;47(1):195-204. Epub 2007 Dec 6. PMID:18052346[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Silva MS, Barata L, Ferreira AE, Romao S, Tomas AM, Freire AP, Cordeiro C. Catalysis and structural properties of Leishmania infantum glyoxalase II: trypanothione specificity and phylogeny. Biochemistry. 2008 Jan 8;47(1):195-204. Epub 2007 Dec 6. PMID:18052346 doi:10.1021/bi700989m