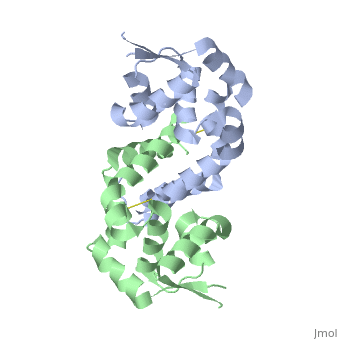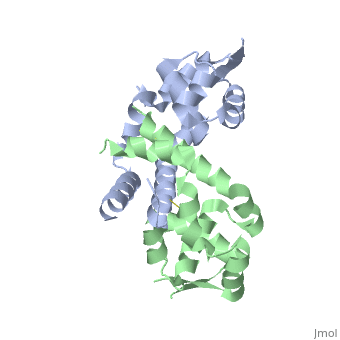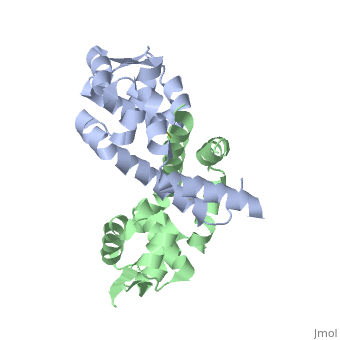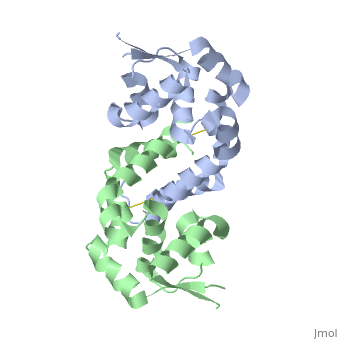
Structural highlights
Function
Q93R11_XANCH
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
The Xanthomonas campestris transcription regulator OhrR contains a reactive cysteine residue (C22) that upon oxidation by organic hydroperoxides (OHPs) forms an intersubunit disulphide bond with residue C127'. Such modification induces the expression of a peroxidase that reduces OHPs to their less toxic alcohols. Here, we describe the structures of reduced and OHP-oxidized OhrR, visualizing the structural mechanism of OHP induction. Reduced OhrR takes a canonical MarR family fold with C22 and C127' separated by 15.5 A. OHP oxidation results in the disruption of the Y36'-C22-Y47' interaction network and dissection of helix alpha5, which then allows the 135 degrees rotation and 8.2 A translation of C127', formation of the C22-C127' disulphide bond, and alpha6-alpha6' helix-swapped reconfiguration of the dimer interface. These changes result in the 28 degrees rigid body rotations of each winged helix-turn-helix motif and DNA dissociation. Similar effector-induced rigid body rotations are expected for most MarR family members.
Structural mechanism of organic hydroperoxide induction of the transcription regulator OhrR.,Newberry KJ, Fuangthong M, Panmanee W, Mongkolsuk S, Brennan RG Mol Cell. 2007 Nov 30;28(4):652-64. PMID:18042459[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Newberry KJ, Fuangthong M, Panmanee W, Mongkolsuk S, Brennan RG. Structural mechanism of organic hydroperoxide induction of the transcription regulator OhrR. Mol Cell. 2007 Nov 30;28(4):652-64. PMID:18042459 doi:10.1016/j.molcel.2007.09.016