Structural highlights
Function
[PHOP_ECOLI] Member of the two-component regulatory system PhoP/PhoQ involved in adaptation to low Mg(2+) environments and the control of acid resistance genes. In low periplasmic Mg(2+), PhoQ phosphorylates PhoP, resulting in the expression of PhoP-activated genes (PAG) and repression of PhoP-repressed genes (PRG). In high periplasmic Mg(2+), PhoQ dephosphorylates phospho-PhoP, resulting in the repression of PAG and may lead to expression of some PRG (By similarity). Mediates magnesium influx to the cytosol by activation of MgtA. Promotes expression of the two-component regulatory system rstA/rstB and transcription of the hemL, mgrB, nagA, slyB, vboR and yrbL genes.[1]
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
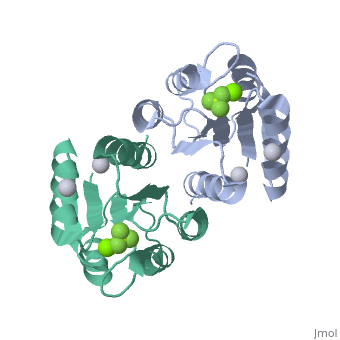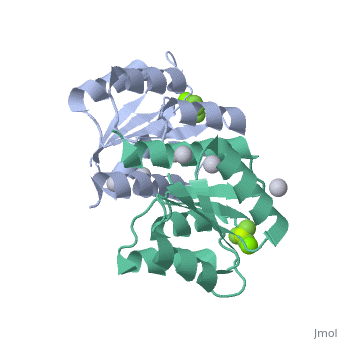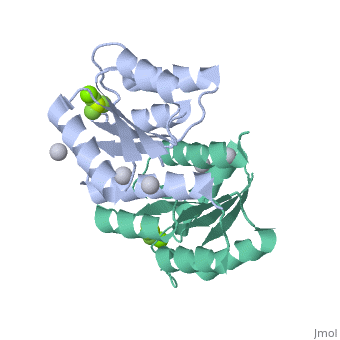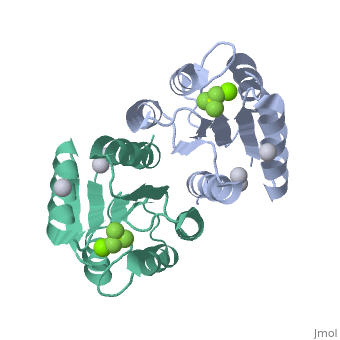
The response regulator PhoP is part of the PhoQ/PhoP two-component system involved in responses to depletion of extracellular Mg(2+). Here, we report the crystal structures of the receiver domain of Escherichia coli PhoP determined in the absence and presence of the phosphoryl analog beryllofluoride. In the presence of beryllofluoride, the active receiver domain forms a twofold symmetric dimer similar to that seen in structures of other regulatory domains from the OmpR/PhoB family, providing further evidence that members of this family utilize a common mode of dimerization in the active state. In the absence of activating agents, the PhoP receiver domain crystallizes with a similar structure, consistent with the previous observation that high concentrations can promote an active state of PhoP independent of phosphorylation.
Crystal structures of the receiver domain of the response regulator PhoP from Escherichia coli in the absence and presence of the phosphoryl analog beryllofluoride.,Bachhawat P, Stock AM J Bacteriol. 2007 Aug;189(16):5987-95. Epub 2007 Jun 1. PMID:17545283[2]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Kato A, Tanabe H, Utsumi R. Molecular characterization of the PhoP-PhoQ two-component system in Escherichia coli K-12: identification of extracellular Mg2+-responsive promoters. J Bacteriol. 1999 Sep;181(17):5516-20. PMID:10464230
- ↑ Bachhawat P, Stock AM. Crystal structures of the receiver domain of the response regulator PhoP from Escherichia coli in the absence and presence of the phosphoryl analog beryllofluoride. J Bacteriol. 2007 Aug;189(16):5987-95. Epub 2007 Jun 1. PMID:17545283 doi:10.1128/JB.00049-07