2rd0
From Proteopedia
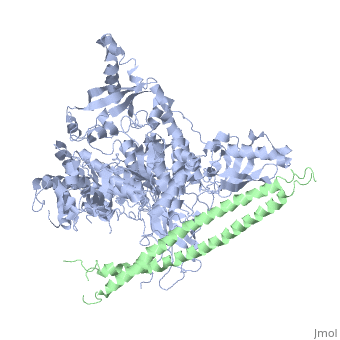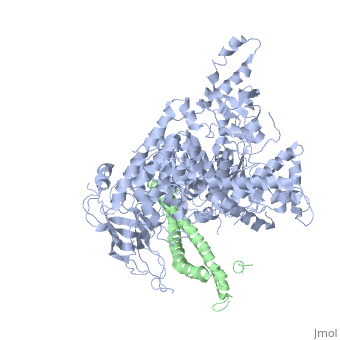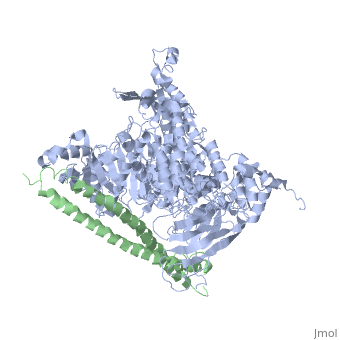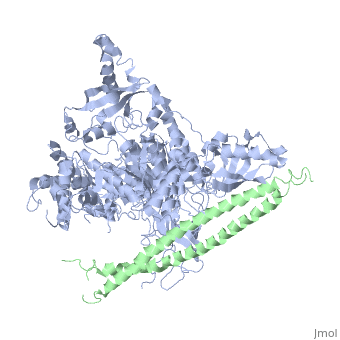
Structure of a human p110alpha/p85alpha complex
Structural highlights
DiseasePK3CA_HUMAN Note=Most of the cancer-derived mutations are missense mutations and map to one of the three hotspots: Glu-542; Glu-545 and His-1047. Mutated isoforms participate in cellular transformation and tumorigenesis induced by oncogenic receptor tyrosine kinases (RTKs) and HRAS1/KRAS. Interaction with HRAS1/KRAS is required for Ras-driven tumor formation. Mutations increasing the lipid kinase activity are required for oncogenic signaling. The protein kinase activity may not be required for tumorigenesis. Defects in PIK3CA are associated with colorectal cancer (CRC) [MIM:114500. Defects in PIK3CA are a cause of susceptibility to breast cancer (BC) [MIM:114480. A common malignancy originating from breast epithelial tissue. Breast neoplasms can be distinguished by their histologic pattern. Invasive ductal carcinoma is by far the most common type. Breast cancer is etiologically and genetically heterogeneous. Important genetic factors have been indicated by familial occurrence and bilateral involvement. Mutations at more than one locus can be involved in different families or even in the same case. Defects in PIK3CA are a cause of susceptibility to ovarian cancer (OC) [MIM:167000. Ovarian cancer common malignancy originating from ovarian tissue. Although many histologic types of ovarian neoplasms have been described, epithelial ovarian carcinoma is the most common form. Ovarian cancers are often asymptomatic and the recognized signs and symptoms, even of late-stage disease, are vague. Consequently, most patients are diagnosed with advanced disease. Defects in PIK3CA may underlie hepatocellular carcinoma (HCC) [MIM:114550.[1] Defects in PIK3CA are a cause of keratosis seborrheic (KERSEB) [MIM:182000. A common benign skin tumor. Seborrheic keratoses usually begin with the appearance of one or more sharply defined, light brown, flat macules. The lesions may be sparse or numerous. As they initially grow, they develop a velvety to finely verrucous surface, followed by an uneven warty surface with multiple plugged follicles and a dull or lackluster appearance.[2] Defects in PIK3CA are the cause of congenital lipomatous overgrowth, vascular malformations, and epidermal nevi (CLOVE) [MIM:612918. CLOVE is a sporadically occurring, non-hereditary disorder characterized by asymmetric somatic hypertrophy and anomalies in multiple organs. It is defined by four main clinical findings: congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and skeletal/spinal abnormalities. The presence of truncal overgrowth and characteristic patterned macrodactyly at birth differentiates CLOVE from other syndromic forms of overgrowth.[3] FunctionPK3CA_HUMAN Phosphoinositide-3-kinase (PI3K) that phosphorylates PtdIns (Phosphatidylinositol), PtdIns4P (Phosphatidylinositol 4-phosphate) and PtdIns(4,5)P2 (Phosphatidylinositol 4,5-bisphosphate) to generate phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 plays a key role by recruiting PH domain-containing proteins to the membrane, including AKT1 and PDPK1, activating signaling cascades involved in cell growth, survival, proliferation, motility and morphology. Participates in cellular signaling in response to various growth factors. Involved in the activation of AKT1 upon stimulation by receptor tyrosine kinases ligands such as EGF, insulin, IGF1, VEGFA and PDGF. Involved in signaling via insulin-receptor substrate (IRS) proteins. Essential in endothelial cell migration during vascular development through VEGFA signaling, possibly by regulating RhoA activity. Required for lymphatic vasculature development, possibly by binding to RAS and by activation by EGF and FGF2, but not by PDGF. Regulates invadopodia formation in breast cancer cells through the PDPK1-AKT1 pathway. Participates in cardiomyogenesis in embryonic stem cells through a AKT1 pathway. Participates in vasculogenesis in embryonic stem cells through PDK1 and protein kinase C pathway. Has also serine-protein kinase activity: phosphorylates PIK3R1 (p85alpha regulatory subunit), EIF4EBP1 and HRAS.[4] Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. See AlsoReferences
| ||||||||||||||||||