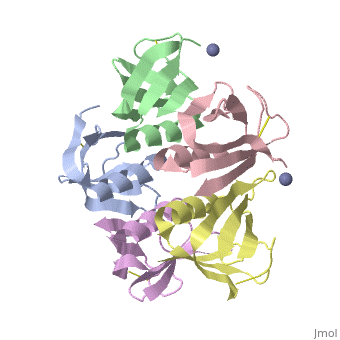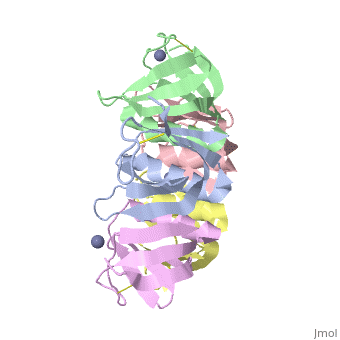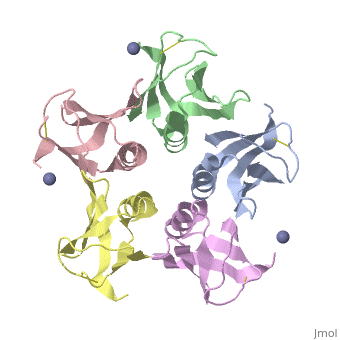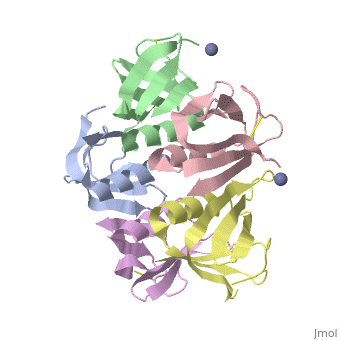Structural highlights
Function
STXB_BPH19 The B subunit is responsible for the binding of the holotoxin to specific receptors on the target cell surface, such as globotriaosylceramide (Gb3) in human intestinal microvilli.
Publication Abstract from PubMed
The Shiga toxin family, a group of cytotoxins associated with diarrhoeal diseases and the haemolytic uraemic syndrome, includes Shiga toxin from Shigella dysenteriae type 1 and verotoxins produced by enteropathogenic Escherichia coli. The family belongs to the A-B class of bacterial toxins, which includes the cholera toxin family, pertussis and diphtheria toxins. These toxins all have bipartite structures consisting of an enzymatic A subunit associated with a B oligomer which binds to specific cell-surface receptors, but their amino-acid sequences and pathogenic mechanisms differ. We have determined the crystal structure of the B oligomer of verotoxin-1 from E. coli. The structure unexpectedly resembles that of the B oligomer of the cholera toxin-like heat-labile enterotoxin from E. coli, despite the absence of detectable sequence similarity between these two proteins. This result implies a distant evolutionary relationship between the Shiga toxin and cholera toxin families. We suggest that the cell surface receptor-binding site lies in a cleft between adjacent subunits of the B pentamer, providing a potential target for drugs and vaccines to prevent toxin binding and effect.
Crystal structure of the cell-binding B oligomer of verotoxin-1 from E. coli.,Stein PE, Boodhoo A, Tyrrell GJ, Brunton JL, Read RJ Nature. 1992 Feb 20;355(6362):748-50. PMID:1741063[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Stein PE, Boodhoo A, Tyrrell GJ, Brunton JL, Read RJ. Crystal structure of the cell-binding B oligomer of verotoxin-1 from E. coli. Nature. 1992 Feb 20;355(6362):748-50. PMID:1741063 doi:http://dx.doi.org/10.1038/355748a0