Structural highlights
Function
TIRAP_HUMAN Adapter involved in TLR2 and TLR4 signaling pathways in the innate immune response. Acts via IRAK2 and TRAF-6, leading to the activation of NF-kappa-B, MAPK1, MAPK3 and JNK, and resulting in cytokine secretion and the inflammatory response. Positively regulates the production of TNF-alpha and interleukin-6.[1] [2]
Publication Abstract from PubMed
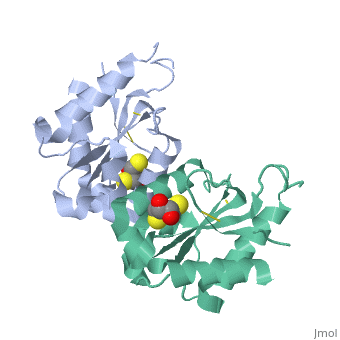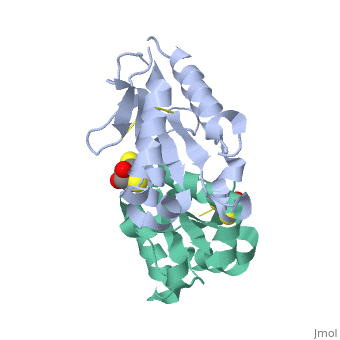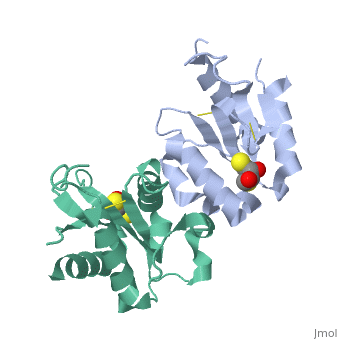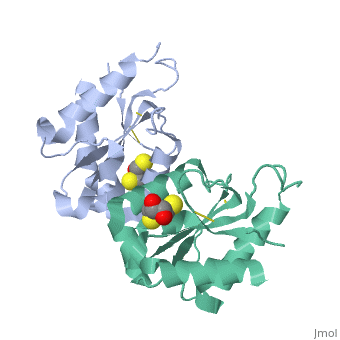
MyD88 adaptor-like protein (Mal) is a crucial adaptor that acts as a bridge to recruit the MyD88 molecule to activated TLR4 receptors in response to invading pathogens. The specific assembly of the Toll/interleukin-1 receptor (TIR) domains of TLR4, Mal and MyD88 is responsible for proper signal transduction in the TLR4 signaling pathway. However, the molecular mechanism for the specificity of these TIR domains remains unclear. Here, we present the crystal structure of the TIR domain of the human Mal molecule (Mal-TIR) at a resolution of 2.4 A. Unexpectedly, Mal-TIR exhibits an extraordinarily long AB loop, but no alphaB helix or BB loop, distinguishing it from other TIR domains. More importantly, the Mal-TIR AB loop is capable of mediating direct binding to the TIR domains of TLR4 and MyD88 simultaneously. We also found that Mal-TIR can form a back-to-back dimer that may resemble the dimeric assembly of the entire Mal molecule. Our data demonstrate the bridge role of the Mal-TIR domain and provide important information about TIR domain specificity.
Structural insights into TIR domain specificity of the bridging adaptor Mal in TLR4 signaling.,Lin Z, Lu J, Zhou W, Shen Y PLoS One. 2012;7(4):e34202. Epub 2012 Apr 2. PMID:22485159[3]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Semaan N, Alsaleh G, Gottenberg JE, Wachsmann D, Sibilia J. Etk/BMX, a Btk family tyrosine kinase, and Mal contribute to the cross-talk between MyD88 and FAK pathways. J Immunol. 2008 Mar 1;180(5):3485-91. PMID:18292575
- ↑ Nagpal K, Plantinga TS, Wong J, Monks BG, Gay NJ, Netea MG, Fitzgerald KA, Golenbock DT. A TIR domain variant of MyD88 adapter-like (Mal)/TIRAP results in loss of MyD88 binding and reduced TLR2/TLR4 signaling. J Biol Chem. 2009 Sep 18;284(38):25742-8. doi: 10.1074/jbc.M109.014886. Epub 2009, Jun 9. PMID:19509286 doi:http://dx.doi.org/10.1074/jbc.M109.014886
- ↑ Lin Z, Lu J, Zhou W, Shen Y. Structural insights into TIR domain specificity of the bridging adaptor Mal in TLR4 signaling. PLoS One. 2012;7(4):e34202. Epub 2012 Apr 2. PMID:22485159 doi:10.1371/journal.pone.0034202