4mqe
From Proteopedia
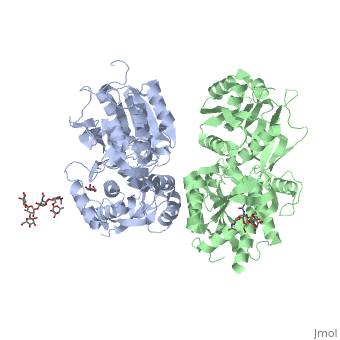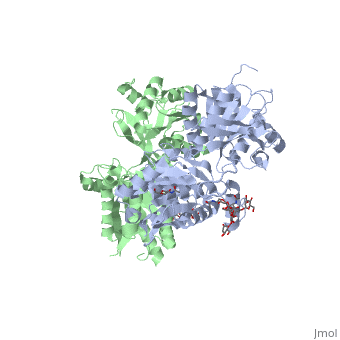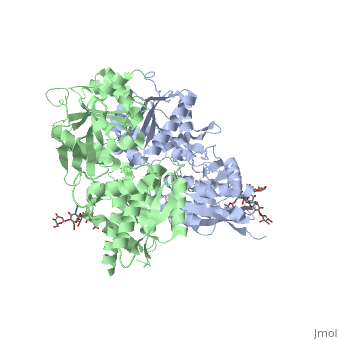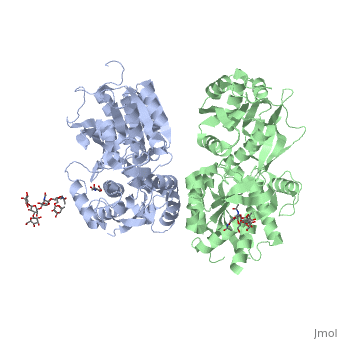
Crystal structure of the extracellular domain of human GABA(B) receptor in the apo form
Structural highlights
FunctionGABR1_HUMAN Component of a heterodimeric G-protein coupled receptor for GABA, formed by GABBR1 and GABBR2. Within the heterodimeric GABA receptor, only GABBR1 seems to bind agonists, while GABBR2 mediates coupling to G proteins. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Signaling inhibits adenylate cyclase, stimulates phospholipase A2, activates potassium channels, inactivates voltage-dependent calcium-channels and modulates inositol phospholipid hydrolysis. Calcium is required for high affinity binding to GABA. Plays a critical role in the fine-tuning of inhibitory synaptic transmission. Pre-synaptic GABA receptor inhibits neurotransmitter release by down-regulating high-voltage activated calcium channels, whereas postsynaptic GABA receptor decreases neuronal excitability by activating a prominent inwardly rectifying potassium (Kir) conductance that underlies the late inhibitory postsynaptic potentials. Not only implicated in synaptic inhibition but also in hippocampal long-term potentiation, slow wave sleep, muscle relaxation and antinociception. Activated by (-)-baclofen, cgp27492 and blocked by phaclofen.[1] [2] [3] [4] Isoform 1E may regulate the formation of functional GABBR1/GABBR2 heterodimers by competing for GABBR2 binding. This could explain the observation that certain small molecule ligands exhibit differential affinity for central versus peripheral sites.[5] [6] [7] [8] Publication Abstract from PubMedHuman GABA(B) (gamma-aminobutyric acid class B) receptor is a G-protein-coupled receptor central to inhibitory neurotransmission in the brain. It functions as an obligatory heterodimer of the subunits GBR1 and GBR2. Here we present the crystal structures of a heterodimeric complex between the extracellular domains of GBR1 and GBR2 in the apo, agonist-bound and antagonist-bound forms. The apo and antagonist-bound structures represent the resting state of the receptor; the agonist-bound complex corresponds to the active state. Both subunits adopt an open conformation at rest, and only GBR1 closes on agonist-induced receptor activation. The agonists and antagonists are anchored in the interdomain crevice of GBR1 by an overlapping set of residues. An antagonist confines GBR1 to the open conformation of the inactive state, whereas an agonist induces its domain closure for activation. Our data reveal a unique activation mechanism for GABA(B) receptor that involves the formation of a novel heterodimer interface between subunits. Structural mechanism of ligand activation in human GABA(B) receptor.,Geng Y, Bush M, Mosyak L, Wang F, Fan QR Nature. 2013 Dec 12;504(7479):254-9. doi: 10.1038/nature12725. Epub 2013 Dec 4. PMID:24305054[9] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
| ||||||||||||||||||||
Categories: Homo sapiens | Large Structures | Bush M | Fan QR | Geng Y | Mosyak L | Wang F