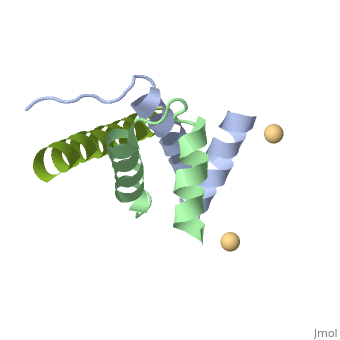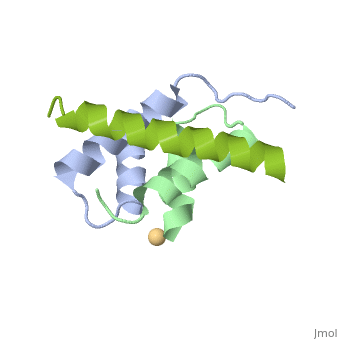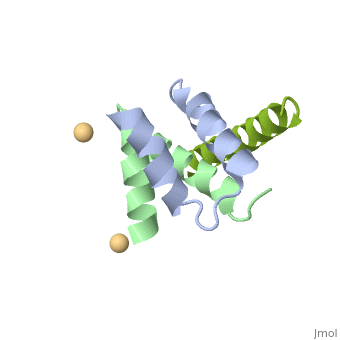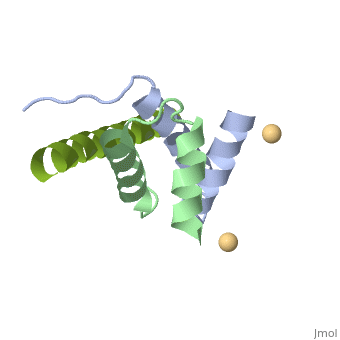
Structural highlights
Function
AKA7G_HUMAN Probably targets cAMP-dependent protein kinase (PKA) to the cellular membrane or cytoskeletal structures. The membrane-associated form reduces epithelial sodium channel (ENaC) activity, whereas the free cytoplasmic form may negatively regulate ENaC channel feedback inhibition by intracellular sodium.[1] [2]
Publication Abstract from PubMed
A-kinase anchoring proteins (AKAPs) interact with the dimerization/docking (D/D) domains of regulatory subunits of the ubiquitous protein kinase A (PKA). AKAPs tether PKA to defined cellular compartments establishing distinct pools to increase the specificity of PKA signalling. Here, we elucidated the structure of an extended PKA-binding domain of AKAP18beta bound to the D/D domain of the regulatory RIIalpha subunits of PKA. We identified three hydrophilic anchor points in AKAP18beta outside the core PKA-binding domain, which mediate contacts with the D/D domain. Such anchor points are conserved within AKAPs that bind regulatory RII subunits of PKA. We derived a different set of anchor points in AKAPs binding regulatory RI subunits of PKA. <em>In vitro</em> and cell-based experiments confirm the relevance of these sites for the interaction of RII subunits with AKAP18 and of RI subunits with the RI-specific smAKAP. Thus we report a novel mechanism governing interactions of AKAPs with PKA. The sequence specificity of each AKAP around the anchor points and the requirement of these points for the tight binding of PKA allow the development of selective inhibitors to unequivocally ascribe cellular functions to the AKAP18-PKA and other AKAP-PKA interactions.
AKAP18:PKA-RIIalpha structure reveals crucial anchor points for recognition of regulatory subunits of PKA.,Gotz F, Roske Y, Schulz MS, Autenrieth K, Bertinetti D, Faelber K, Zuhlke K, Kreuchwig A, Kennedy E, Krause G, Daumke O, Herberg FW, Heinemann U, Klussmann E Biochem J. 2016 Apr 21. pii: BCJ20160242. PMID:27102985[3]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Trotter KW, Fraser ID, Scott GK, Stutts MJ, Scott JD, Milgram SL. Alternative splicing regulates the subcellular localization of A-kinase anchoring protein 18 isoforms. J Cell Biol. 1999 Dec 27;147(7):1481-92. PMID:10613906
- ↑ Bengrine A, Li J, Awayda MS. The A-kinase anchoring protein 15 regulates feedback inhibition of the epithelial Na+ channel. FASEB J. 2007 Apr;21(4):1189-201. Epub 2007 Jan 23. PMID:17244820 doi:http://dx.doi.org/10.1096/fj.06-6046com
- ↑ Gotz F, Roske Y, Schulz MS, Autenrieth K, Bertinetti D, Faelber K, Zuhlke K, Kreuchwig A, Kennedy E, Krause G, Daumke O, Herberg FW, Heinemann U, Klussmann E. AKAP18:PKA-RIIalpha structure reveals crucial anchor points for recognition of regulatory subunits of PKA. Biochem J. 2016 Apr 21. pii: BCJ20160242. PMID:27102985 doi:http://dx.doi.org/10.1042/BCJ20160242