Introduction
Discovered and isolated by Anselme Payen in 1833, amylase was the first enzyme to be discovered[1]. Amylases are hydrolases, acting on α-1,4-glycosidic bonds[2]. They can be further subdivided into α,β and γ amylases[1].α-Amylase (AAM) is an enzyme that acts as a catalyst for the hydrolysis of α-linked polysaccharides into α-anomeric products[3]. The enzyme can be derived from a variety of sources, each with different characteristics. α-Amylase found within the human body serves as the enzyme active in pancreatic juice and saliva[2]. α-Amylase is not only essential in human physiology but has a number of important biotechnological functions in various processing industries. β/α amylase (BAAM) is a precursor protein which is cleaved to form the β-amylase and α-amylase after secretion. β amylase (BAM) acts at the non-reducing chain ends and liberate only β-maltose[4]. γ amylase (GAM) acts at the non-reducing chain ends of amylose and amylopectin and liberates glucose. Pullulanase hydrolyses the α-1,6 glucoside linkage in starch, amylopectin, pullulan and related oligosaccharides[5].
- Neopullulanase is involved in starch degrading[6].
For α-amylase see Raghad zoubi
See also Amylase (Hebrew).
Structure[3]
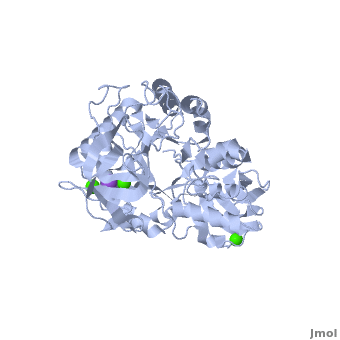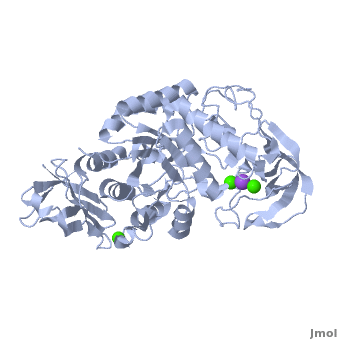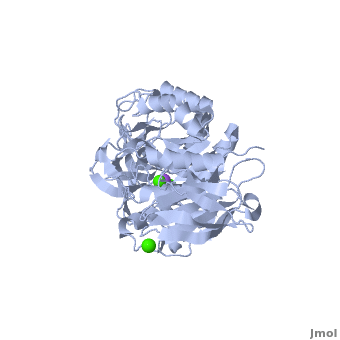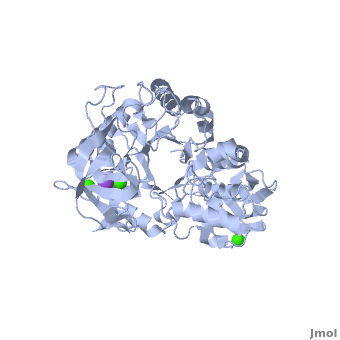
Shown as 1hvx is the structure of the thermostable α-amylase of Bacillus stearothermophilus (BSTA)[3]. BSTA is comprised of a single polypeptide chain. This chain is folded into three domains: A, B and C. These domains are generally found on all α-amylase enzymes. The constitutes the core structure, with a (β/α)8-barrel.The consists of a sheet of four anti-parallel β-strands with a pair of anti-parallel β-strands. Long loops are observed between the β-strands. Located within the B domain is the for Ca2+-Na+-Ca2+. consisting of eight β-strands is assembled into a globular unit forming a Greek key motif. It also holds the Ca2+ binding site in association with domain A. Positioned on the C-terminal side of the β-strands of the (β/α)8-barrel in domain A is the active site. The catalytic residues involved for the BSTA active site are . The residues are identical to other α-amylases, yet there are positional differences which reflect the flexible nature of catalytic resides.
found in the interior of domain B and at the interface of domain A and C, constitute the metal ion binding sites. All α-amylases contain one strongly conserved Ca2+ ion for structural integrity and enzymatic activity.[7] CaI is consistent in α-amylases, however there are structural differences between the linear trio of CaI, CaII and Na in other enzymes. CaIII acts as a bridge between two loops, one from α6 of domain A, and one between β1 and β2 of domain C.
Chloride Dependent Enzymes
A family of chloride-dependent enzymes, including salivary and pancreatic α-amylase, require the binding of a chloride ion to be allosterically activated[7]. The function of the chloride ion still remains uncertain. No relationship has been observed between the anion binding affinity and its activity, indicating the complexity between the binding parameters and mechanism it activates[7]. Studies have shown that nitrite and nitrate ions with pancreatic α-amylase fit within the chloride binding site, thus making all the necessary hydrogen bonds and enhancing the relative activity by 5-fold[8].
Function
Mechanism
In the human body, α-amylase is part of digestion with the breakdown of carbohydrates in the diet. The mechanism involved includes catalyzing substrate hydrolysis by a double replacement mechanism, forming a covalent glycosyl-enzyme intermediate and hydrolyzed through oxocarbenium ion-like transition states[9]. One of the carboxylic acids in the active site acts as the catalytic nucleophile during the formation of the intermediate. A second carboxylic acid operates as the acid/base catalyst, supporting the stabilization of the transition states during the hydrolysis[9].
Human Salivary and Pancreatic α-Amylase
Salivary α-Amylase hydrolyzes the (α1-4) glycosidic linkages of starch, separating it into short polysaccharide fragments[10]. Once the enzyme reaches the stomach, it becomes inactivated due to the acidic pH. Further breakdown of starch occurs by secretion of a second form of the enzyme by the pancreas. Pancreatic juice enters the duodenum and pancreatic α-amylase further cleaves starch to yield maltose, maltotriose and oligosaccharides[10]. The oligosaccharides are referred to as dextrins, which are fragments of amylopectin consisting of (α1-6)branch points[10]. Microvilli of the intestinal epithelia break maltose and dextrins into glucose, which gets absorbed into the circulatory system[10]. Glycogen has a relatively similar structure as starch, and thus proceeds in the same digestive pathway.
Regulation
α-Amylase is regulated through a number of inhibitors. These inhibitors are classified according to six categories, based on their tertiary structures[11]. Inhibitors of α-amylase block the active site of the enzyme. In animals, inhibitors control the conversion of starch to simple sugars during glucose peaks after a meal so that breakdown of glucose occurs at a rate the body can handle[11]. This is particularly important for diabetics, who require low quantities of α-amylase to maintain control over glucose levels. After taking insulin however, pancreatic α-amylase escalates. Plants use these inhibitors as a defense mechanism to inhibit the use of α-amylase in insects, thus protecting themselves from herbivory[12].
Industrial Uses
α-Amylase is used extensively in various industrial processes. In textile weaving, starch is added for warping. After weaving, the starch is removed by Bacillus subtilis α-amylase[1]. Dextrin, which is a viscosity improver, filler, or ingredient of food, is manufactured by the liquefaction of starch by bacteria α-amylase[1]. Bacterial α-amylases of B.subtilis, or B.licheniformis are used for the initial starch liquefaction in producing high conversion glucose syrup[1]. Pancreatitis can be tested by determining the level of amylases in the blood, a result of damaged amylase-producing cells, or excretion due to renal failure[13]. α-Amylase is used for the production of malt, as the enzyme is produced during the germination of cereal grains[1].
β/α amylase (BAAM) is a precursor protein which is cleaved to form the β-amylase and α-amylase after secretion.
Structure of the AmyC GH13 alpha-amylase from Alicyclobacillus sp, reveals accommodation of starch branching points in the alpha-amylase family[14]
The enzymatic degradation of starch has a myriad industrial applications. However, the branched nature of the polysaccharides that compose it poses problems, as branches have to be accommodated within an active centre best suited to linear polysaccharides. Alpha-amylases are glycoside hydrolases that break the α-1,4 bonds in starch and related glycans. The present work provides a rare insight into branch-point acceptance in these industrial catalysts.
The complex of α-amylase from Alicyclobacillus sp. 18711 (AliC) with acarbose was solved by molecular replacement, with two molecules of AliC in the asymmetric unit, at a resolution of 2.1 Å (6gxv). The fold, as expected, is a canonical with the A, B and C domains defined approximately as A, residues 4–104 and 210–397 (in deepskyblue), B, residues 105–209 (in yellow), and C, residues 398–484 (in white). A classical Ca2+–Na+–Ca2+ [15],[16] is found at the A/B-domain interface. The structure of AliC was determined in the presence of the (colored in green). As with many (retaining) α-amylase complexes, the acarbose is observed as a transglycosylated species, here a hexasaccharide which contains two of the acarviosin disaccharide motifs. The , -4 to +2, with the expected catalytic GH13 signature triad of Asp234 (nucleophile), Glu265 (acid/base) and all disposed for catalysis, here around the 2H3 half-chair of the unsaturated cyclohexitol moiety. AliC must also be able to accommodate branching in the +2 subsite, which is consistent with the .
A ‘branched-ligand’ AliC complex was obtained through co-crystallization, with crystals forming in a new space group. This form diffracted poorly and data could only be obtained to 2.95 Å resolution 6gya). Weak density in the -1 subsite, largely diffuse but greater than would be expected for discrete solvent, remained unmodelled. Density was clearer for a panose trisaccharide with an α-1,4-linked disaccharide in subsites +1 and +2 and, crucially, clear density for an α-1,6 branch accommodated in the +1 subsite, providing a structural context for the limit digest analysis of action on amylopectin starch. The (oligosaccharide colored in green).
3D structures of amylase
Amylase 3D structures