α-Amylase is a protein enzyme EC 3.2.1.1 that hydrolyses alpha bonds of large, alpha-linked polysaccharides, such as starch and glycogen, yielding glucose and maltose. It is the major form of amylase found in humans and other mammals.It is also present in seeds containing starch as a food reserve, and is secreted by many fungi. [1]
Introduction
The (EC 3.2.1.1 ) (CAS# 9014-71-5) (alternative names: 1,4-α-D-glucan glucanohydrolase; glycogenase) are calcium metalloenzymes, completely unable to function in the absence of calcium. By acting at random locations along the starch chain, α-amylase breaks down long-chain carbohydrates, ultimately yielding maltotriose and maltose from amylose, or maltose, glucose and "limit dextrin" from amylopectin. Because it can act anywhere on the substrate, α-amylase tends to be faster-acting than β-amylase. In animals, it is a major digestive enzyme, and its optimum pH is 6.7–7.0
In human physiology, both the salivary and pancreatic amylases are α-amylases.
The α-amylases form is also found in plants, fungi (ascomycetes and basidiomycetes) and bacteria (Bacillus) [2]
Industrial use
α-Amylase is used in ethanol production to break starches in grains into fermentable sugars.
The first step in the production of high-fructose corn syrup is the treatment of cornstarch with α-amylase, producing shorter chains of sugars oligosaccharides.
An α-amylase called "Termamyl", sourced from Bacillus licheniformis, is also used in some detergents, especially dishwashing and starch-removing detergents.[3]
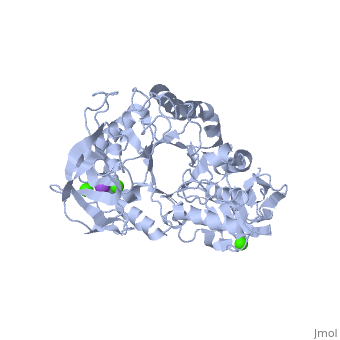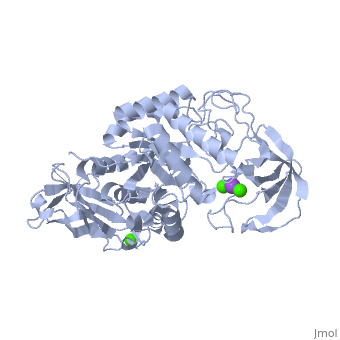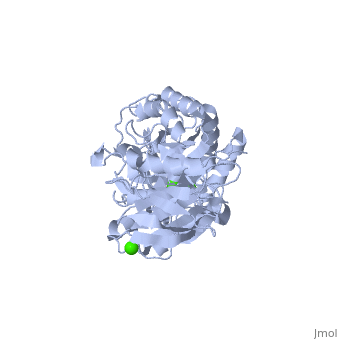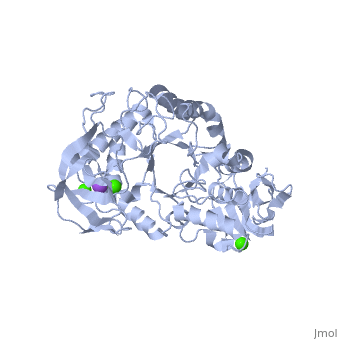
Structural highlights
Shown as 1hvx is the structure of the thermostable α-amylase of Bacillus stearothermophilus (BSTA)[3]. BSTA is comprised of a single polypeptide chain. This chain is folded into three domains: A, B and C. These domains are generally found on all α-amylase enzymes. The constitutes the core structure, with a (β/α)8-barrel.The consists of a sheet of four anti-parallel β-strands with a pair of anti-parallel β-strands. Long loops are observed between the β-strands. Located within the B domain is the binding site for Ca2+-Na+-Ca2+. consisting of eight β-strands is assembled into a globular unit forming a Greek key motif. It also holds the third Ca2+ binding site in association with domain A. Positioned on the C-terminal side of the β-strands of the (β/α)8-barrel in domain A is the active site. The catalytic residues involved for the BSTA active site are.The residues are identical to other α-amylases, yet there are positional differences which reflect the flexible nature of catalytic resides. found in the interior of domain B and CaIII at the interface of domain A and C, constitute the metal ion binding sites. All α-amylases contain one strongly conserved Ca2+ ion for structural integrity and enzymatic activity.[4] CaI is consistent in α-amylases, however there are structural differences between the linear trio of CaI, CaII and Na in other enzymes. CaIII acts as a bridge between two loops, one from α6 of domain A, and one between β1 and β2 of domain C.