Chaperones are proteins that are involved in the folding and unfolding of other macromolecules. The word chaperone means 'giving protection' which implies the idea of preventing new proteins from misfolding. They exists both in prokaryotes and eukaryotes. Some chaperones are constitutively expressed in the system where as other chaperones are expressed only in response to an external stimulus or stress such as heat and therefore they are referred to as heat shock proteins. They are classified based on their structure, size, molecular weight and function in to several classes such as Hsp40s, Hsp60s Chaperonin, , Hsp90s such as 1am1, Hsp100s and small heat shock proteins like (alpha)-crystallin proteins. Various classes of molecular chaperones cooperate for the folding of the nascent polypeptide chains in the cyotplasm [1]. See also Heat Shock Proteins.
Function
Chaperones bind to the newly synthesized and unfolded proteins helping them acquire their properly folded 3D structure [2]. Besides, chaperones help in targeting the native proteins to their respective organelles [3][4]. The first identified chaperones were the histone chaperones that are continously involved in histone metabolism thus regulating genome function, stability and identity[5]. Many protozoan parasites such as Plasmodium falciparum requires these proteins for cytoprotection and targeting of nuclear encoded proteins to apicoplast required for necessary biosynthetic processes like fatty acid biosynthesis [6][7]. Chaperones actively participate in the maintenance of proteome integrity and protein homeostasis (proteostasis) which requires a syncrhonization in various chaperones tuning the process [8].
Disease
Chaperones are instrumental in protein folding processes. Alteration in this process may lead to protein aggregation and formation of inclusion bodies. Protein misfolding may result in various diseases such as Alzheimer [9], Parkinson [10], Familial amyotrophic lateral sclerosis [11], Huntington[12], Spinocerebellar ataxia 1, 2, 3 [13], Spinobulbar muscular atrophy [14] and ageing [15].
Relevance
Modulation of chaperone's expression is a new therapeutic approach for the neurodegenerative and other diseases arising from protein misfolding. There is a distinct network of chaperones and co chaperones that either directly influences the substrate proteins or in association with the protein degradation pathways such as the ubiquitin-proteasome-system or autophagy, results in the removal of completely misfolded and pathogenic proteins[16].
Structural highlights
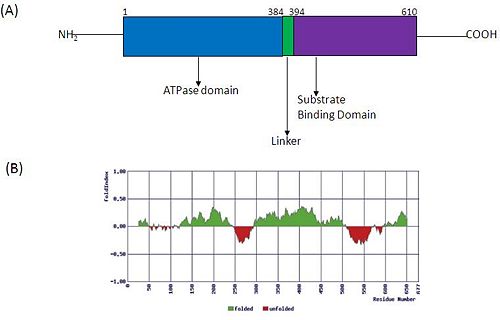
Structurally, hsp70 have a N-terminal followed by a with elongated C-terminal. These domains allosterically regulate the hsp70 functioning. In the 2D figure given below, panel A shows the structural organization of Hsp 70 indicating its various domains. ATP binding and hydrolysis regulates the affinity for substrate proteins which thereafter enhances ATP hydrolysis. Panel B predicts the various folded (green) and unfolded (red) regions in Hsp70.
Structural organization of Hsp70