Clostridium Botulinum Neurotoxin Type B
From Proteopedia
Clostridium Botulinum Neurotoxin Type B
Clostridium Bacteria Family The Clostridium family is a rod shaped bacteria family, which consists of gram positive, anaerobic sulfate reducing bacteria. This family are spore-forming, which makes them relatively resistant to heat. Their spores will not germinate at temperatures below 15 D"C or below pH of 4.6. This family is a big family of bacteria, most of which aren't pathogenic (such as clostridium Acetobutirate). Clostridium Botulinum The Botulinum bacteria is very wide spread (especially its spores); every animal in the ground, in the ocean. The Botulinu bacteria causes a disease by its neurotoxin very rapidly (a few hours). Eating the bacteria as an endospore isn't toxic for adults, but it is for infants under 24 months, because their microbiota is under developed, and cannot compete with the Clostridium Botulinum bacteria endospores. For adults, the neurotoxin is potent when the endospores germinate in contaminated food. For example, swollen tinned food boxes indicate reproduction of the bacteria, and as one opens the tin a sulfate smell will be spread. The Endotoxin The most potent in nature, 1000 time more toxic than Cobra venom. It causes paralysis- muscle relaxation. The muscles are unable to contract due to the toxin's ability to block the secretion of acetylcolin to the synaptic space. The lethal dose (LD) is less than 1 micro gram in humans.
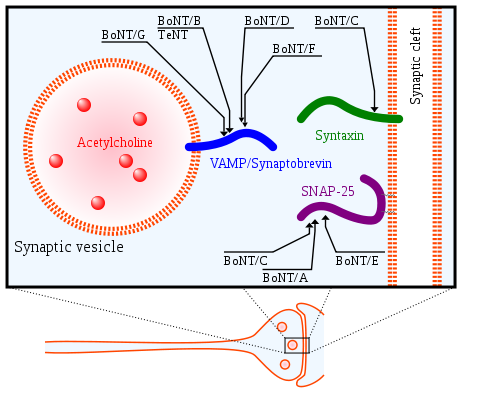
Structural Highlights and FunctionThe toxin size is 150 kDa. In practice the toxin is larger due to creation of complexes with other proteins inside the cell. The pre-protein of the toxin (inactive molecule) undergoes cleavage by a trypsin-like enzyme in the host's GI tract. The cleavage of the separate the toxin into two residues: (1) [50 kDa] which functions as an endopeptidase, (2) [100 kDa] which is composed of two domains. One is the HC- responsible for binding to target cells, and internalization of the toxin molecule into the neuron. The second domain is HN- responsible for the transition of the catalytic residue into the nerve cell cytoplasm. The proteolytic mechanism of botulinum neurotoxins (BoNT/B): The toxin arrives to the synaptic space, then the HC subunit recognizes the nerve cell receptor (possibly due to resembles to the nerve receptor ligand). Endocytosis brings the toxin into the nerve cell in a vesicule. Inside the vesicule the toxin undergoes cleavage into two subunits, the HC and the LC. The LC subunit leaves the vesicule into the cytoplasm and functions as an endopeptidase, which cuts synaptobrevin. Synaptobrevin is a protein responsible for recognition between the vesicule containing acetycoline and the pre-synaptic nerve cell membrane. This causes the acetylcolin not to be released to the synaptic area and prevents muscle contraction. Structural Details: The of the clostridial neurotoxins is the light chain itself. It is centered around a catalytic and structural zinc cation coordinated by a very conserved HEXXH+E motif. In the case of botulinum neurotoxin type B light chain, zinc is directly coordinated by , which are located on the same helix. The fourth ligand is a water molecule that also forms a strong hydrogen bonding interaction with . The side chains from all form 2.1 Å coordinating bonds with the zinc cation. The distance between the water molecule and the zinc cation is 2.2 Å and the water to Glu 230 distance is 2.4 Å. It can be assumed that similar bridging water species would be present in this location in the Synaptobrevin2–botulinum neurotoxin type B light chain substrate complex, to function as an activated nucleophile for attack on the carbonyl carbon of the scissile bond located 3.4 Å away.[1].
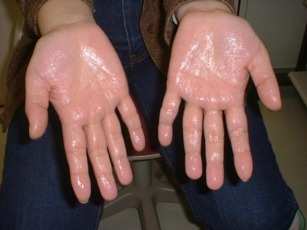
"The proposed catalytic mechanism for BoNT/B is very similar to that of thermolysin, with which BoNT/B-LC shares only weak structural which is characterized by a four-helix bundle. The clostridial neurotoxins are unable to cleave their SNARE substrates once the complex has formed. Proteins in the SNARE family are classified as either Q-SNAREs or R-SNAREs. This classification is based on the presence of either a highly conserved Gln or Arg residue in a structurally critical position in the SNARE complex termed the 'zero layer'. Residues in this position are involved in ionic interactions within the core of the SNARE complex. A repeating pattern of hydrophobic residues from each SNARE protein pack together to form the rest of the SNARE complex core. The neuronal SNARE complex consists of syntaxin and SNAP-25, which both belong to the Q-SNARE family, and synaptobrevin, which is an R-SNARE. This motif of two Q-SNAREs and one R-SNARE per SNARE complex is likely a common occurrence due to the importance of the defining ionic core interactions"[2]. The core Gln/Arg interactions, along with the regular repeating hydrophobic interactions found throughout the core of the SNARE complex, induce a conformational change in synaptobrevin, from almost a random coil in solution to a helical conformation in the SNARE complex. Residues in the SNARE complex that were exposed to the solvent are variable or partially homologous throughout the family and in fact they are the surface residues primarily responsible for the binding interactions between Sb2 and botulinum neurotoxin type B. The scissile bond of synaptobrevin is proximate to the core packing residue of synaptobrevin-2, Phe 77. The access of the botulinum neurotoxin type B light chains to this bond is therefore very restricted once the SNARE complex has formed. The botulinu neurotoxin type B binds a synaptobrevin molecule that is seemingly in a random coil conformation. In contrast, synaptobrevin must exist in helical conformation for SNARE complex formation. Because the toxin's active site lies deeply in the molecule it is unlikely that synaptobrevin can be cleaved in its helical conformation. This stresses out a large entropic advantage for preferential binding of synaptobrevin to the toxin. Once synaptobrevin 2 is bound to the toxin, it is quickly proteolyzed and becomes useless for further participation in SNARE complex formation. The high initial entropic barrier associated with helix formation would result in preferential binding of synaptobrevin to the toxins that proteolyzes it.[3]. DiseaseDisease progress due to food poisoning: Starts with blurry vision because the vision muscles are the highest muscles in the body. Medical care given at this early stage of the poisoning can save from death but the blindness may be irreversible. It is especially dangerous to infants under the age of 2 since they cannot complain about their condition. The next step of the disease progress is relaxation of the mouth muscles- loose tong and jaw, unclear speech and drooling saliva. The neurotoxin doesn't affect the heart muscle thus does not influence blood pressure. The next muscle to be affected is the diaphragm which leads to death by suffocation. RelevanceTreatment of Hyperhidrosis Primary hyperhidrosis, a condition of excess sweating beyond thermoregulatory needs, affects nearly 3% of the US population and carries psychosocial and emotional implications. The highly potent botulinum neurotoxin, derived from Clostridium botulinum bacteria, has been used in a number of medical implications, including minimizing the appearance of facial wrinkles, treatment of cervical dystonia and movement disorders, and relief of hypersalivation and hyperhidrosis. By inhibiting the release of acetylcholine from presynaptic neurons, the botulinum neurotoxin effectively produces paralysis at the neuromuscular junction and similarly inhibits sweat secretion by “chemical denervation”. Although there are seven different botulinum neurotoxin types (A–G), two in particular have been studied for use in humans: type-A, which cleaves the SNAP-25 SNARE protein, and type-B, which cleaves the VAMP SNARE protein. Without SNAP-25 and VAMP SNARE, synaptic vesicles carrying acetylcholine cannot transfuse with the presynaptic neuron cell membrane and release their contents into the synaptic space via exocytosis. In an Italian study of 32 patients by Basciani et al, the efficacy and safety of botulinum neurotoxin type B in the treatment of primary hyperhidrosis were studied. Baseline sweating levels were measured through the Minor’s iodine starch test with weight measurement. The same tests were also performed at 4, 12, and 24 weeks after the neurotoxin was injected. After injection of 5,000 IU of botulinum neurotoxin type B into each palm, reduction of sweating levels from baseline at 4, 12, and 24 weeks in both the right and left palms was statistically significant. Side effects such as local pain and hand weakness were observed in 1/8 of patients and were considered minor. [5].
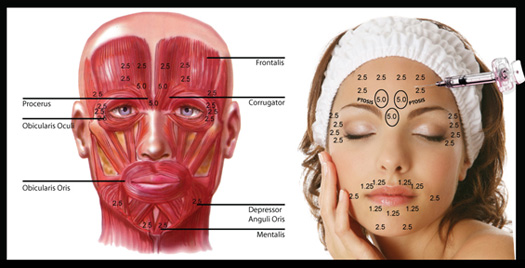
Cosmetic Applications: Facial wrinkles involving the forehead, glabellar, and/or periorbital regions are a common esthetic problem and are directly related to overactivity of the underlying facial muscle layer. For example, the development of glabellar wrinkles is partially related to the dynamics of the underlying procerus, corrugator supercilii, and orbicularis oculi muscles. Certain facial lines are considered especially problematic because they give an appearance of aging. Sometimes they can also be misinterpreted as negative emotions such as anger, anxiety, sadness, fatigue, or stress. In recent years, botulinum neurotoxin injections have become one of the most popular therapies for the treatment of facial wrinkles. After injection, the toxin paralyzes or weakens facial muscles. This reduces or eliminates the appearance of wrinkles and is safe. The paralytical effect of the toxin has been found to be useful in treating a variety of neurologic and nonneurologic disorders. Botulinum neurotoxin type B was approved by the FDA in 2000 for treating cervical dystonia. Several large randomized, placebo-controlled clinical trials have confirmed its safety and efficacy in this patient group. Overall, botulinum neurotoxin type B is a safe and effective therapy for nonsurgically elimination of wrinkles in the upper face. In preliminary dose-response studies, botulinum neurotoxin type B has demonstrated benefit in treating the glabella, forehead, and periorbital region. Studies show that it has a very fast effect of a couple of days. It produces a uniform and smooth paralysis, and exhibits a dose-related response in duration of effect. Further studies are currently conducted to evaluate higher doses of the toxin in order to make the paralysis effects last longer. This novel treatment could benefit patients requesting a fast onset of effect, patients who no longer respond to treatment with botulinum neurotoxin type A (Because the toxins are antigenically distinct, botulinum neurotoxin type A–resistant patients may benefit from treatment with botulinum neurotoxin type B)and patients desiring “touch-up” procedures, because botulinum neurotoxin type B solution does not need to be reconstituted and can be easily stored[7]. Use as a Weapon: Investigations of Clostridium neurotoxin as a biological weapon have been used by many countries. Japan in World War II carried out human experiments on prisoners in Manchuria using the toxin. Also in World War II, Grate britian used a botulism-impregnated grenade in the killing of a German Gestapo officer. The United States researched the botulinum toxin as a military bio-weapon until President Nixon signed the "Biological and Toxin Weapons Convention in 1972", ending all US use in biotoxin as a weapons. Iraq has also weaponize thousands of liters of toxin in warheads after the 1991 Gulf War. The Japanese latest attempt at terrorist use of Clostridium toxin in the early 1990s by Aum Shinryko cult against American military targets, was unsuccessful[9].
| |||||||||||
References
- ↑ Hanson MA, Stevens RC. Cocrystal structure of synaptobrevin-II bound to botulinum neurotoxin type B at 2.0 A resolution. Nat Struct Biol. 2000 Aug;7(8):687-92. PMID:10932255
- ↑ Swaminathan S, Eswaramoorthy S. Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat Struct Biol. 2000 Aug;7(8):693-9. PMID:10932256
- ↑ Segelke B, Knapp M, Kadkhodayan S, Balhorn R, Rupp B. Crystal structure of Clostridium botulinum neurotoxin protease in a product-bound state: Evidence for noncanonical zinc protease activity. Proc Natl Acad Sci U S A. 2004 May 4;101(18):6888-93. Epub 2004 Apr 23. PMID:15107500 doi:10.1073/pnas.0400584101
- ↑ http://en.wikipedia.org/wiki/Botulinum_toxin
- ↑ Stashak AB, Brewer JD. Management of hyperhidrosis. Clin Cosmet Investig Dermatol. 2014 Oct 29;7:285-99. doi: 10.2147/CCID.S53119., eCollection 2014. PMID:25378942 doi:http://dx.doi.org/10.2147/CCID.S53119
- ↑ http://www.medivironuoa.com/2011/08/botox-for-treatment-of-excessive.html
- ↑ Sadick NS. The cosmetic use of botulinum toxin type B in the upper face. Clin Dermatol. 2004 Jan-Feb;22(1):29-33. PMID:15158542 doi:http://dx.doi.org/10.1016/j.clindermatol.2003.12.033
- ↑ http://www.iscgyn.com/en/procedures_botox
- ↑ http://emedicine.medscape.com/article/829125-overview