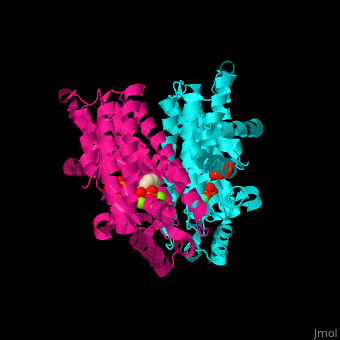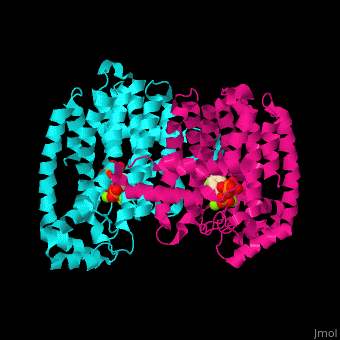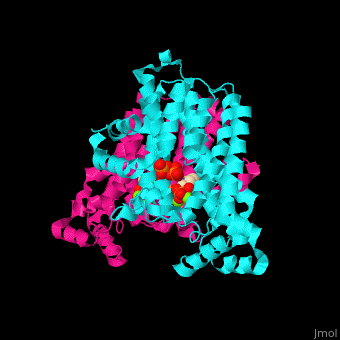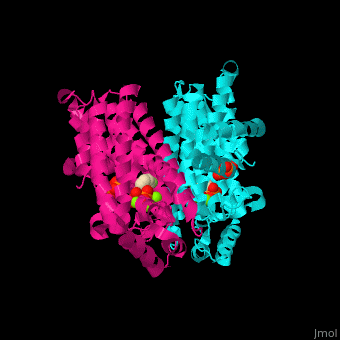We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Farnesyl diphosphate synthase
From Proteopedia
(Redirected from FPPS)
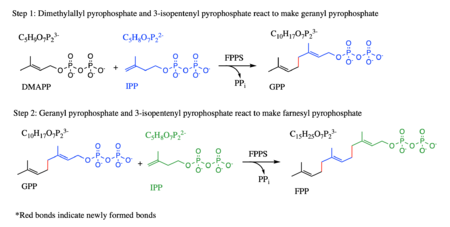
Introduction
| |||||||||||
References
- ↑ Schulbach MC, Mahapatra S, Macchia M, Barontini S, Papi C, Minutolo F, Bertini S, Brennan PJ, Crick DC. Purification, enzymatic characterization, and inhibition of the Z-farnesyl diphosphate synthase from Mycobacterium tuberculosis. J Biol Chem. 2001 Apr 13;276(15):11624-30. Epub 2001 Jan 4. PMID:11152452 doi:http://dx.doi.org/10.1074/jbc.M007168200
- ↑ Aripirala S, Gonzalez-Pacanowska D, Oldfield E, Kaiser M, Amzel LM, Gabelli SB. Structural and thermodynamic basis of the inhibition of Leishmania major farnesyl diphosphate synthase by nitrogen-containing bisphosphonates. Acta Crystallogr D Biol Crystallogr. 2014 Mar;70(Pt 3):802-10. doi:, 10.1107/S1399004713033221. Epub 2014 Feb 22. PMID:24598749 doi:http://dx.doi.org/10.1107/S1399004713033221
- ↑ Mukherjee S, Basu S, Zhang K. Farnesyl pyrophosphate synthase is essential for the promastigote and amastigote stages in Leishmania major. Mol Biochem Parasitol. 2019 Jun;230:8-15. doi: 10.1016/j.molbiopara.2019.03.001. , Epub 2019 Mar 26. PMID:30926449 doi:http://dx.doi.org/10.1016/j.molbiopara.2019.03.001
- ↑ Ortiz-Gomez A, Jimenez C, Estevez AM, Carrero-Lerida J, Ruiz-Perez LM, Gonzalez-Pacanowska D. Farnesyl diphosphate synthase is a cytosolic enzyme in Leishmania major promastigotes and its overexpression confers resistance to risedronate. Eukaryot Cell. 2006 Jul;5(7):1057-64. doi: 10.1128/EC.00034-06. PMID:16835450 doi:http://dx.doi.org/10.1128/EC.00034-06
- ↑ Rodriguez N, Bailey BN, Martin MB, Oldfield E, Urbina JA, Docampo R. Radical cure of experimental cutaneous leishmaniasis by the bisphosphonate pamidronate. J Infect Dis. 2002 Jul 1;186(1):138-40. doi: 10.1086/341074. Epub 2002 Jun 5. PMID:12089677 doi:http://dx.doi.org/10.1086/341074
- ↑ Galaka T, Falcone BN, Li C, Szajnman SH, Moreno SNJ, Docampo R, Rodriguez JB. Synthesis and biological evaluation of 1-alkylaminomethyl-1,1-bisphosphonic acids against Trypanosoma cruzi and Toxoplasma gondii. Bioorg Med Chem. 2019 Aug 15;27(16):3663-3673. doi: 10.1016/j.bmc.2019.07.004., Epub 2019 Jul 4. PMID:31296439 doi:http://dx.doi.org/10.1016/j.bmc.2019.07.004
- ↑ Garzoni LR, Caldera A, Meirelles Mde N, de Castro SL, Docampo R, Meints GA, Oldfield E, Urbina JA. Selective in vitro effects of the farnesyl pyrophosphate synthase inhibitor risedronate on Trypanosoma cruzi. Int J Antimicrob Agents. 2004 Mar;23(3):273-85. doi:, 10.1016/j.ijantimicag.2003.07.020. PMID:15164969 doi:http://dx.doi.org/10.1016/j.ijantimicag.2003.07.020
- ↑ Montalvetti A, Bailey BN, Martin MB, Severin GW, Oldfield E, Docampo R. Bisphosphonates are potent inhibitors of Trypanosoma cruzi farnesyl pyrophosphate synthase. J Biol Chem. 2001 Sep 7;276(36):33930-7. doi: 10.1074/jbc.M103950200. Epub 2001, Jul 2. PMID:11435429 doi:http://dx.doi.org/10.1074/jbc.M103950200
- ↑ Yardley V, Khan AA, Martin MB, Slifer TR, Araujo FG, Moreno SN, Docampo R, Croft SL, Oldfield E. In vivo activities of farnesyl pyrophosphate synthase inhibitors against Leishmania donovani and Toxoplasma gondii. Antimicrob Agents Chemother. 2002 Mar;46(3):929-31. doi:, 10.1128/aac.46.3.929-931.2002. PMID:11850291 doi:http://dx.doi.org/10.1128/aac.46.3.929-931.2002
- ↑ Ling Y, Sahota G, Odeh S, Chan JM, Araujo FG, Moreno SN, Oldfield E. Bisphosphonate inhibitors of Toxoplasma gondi growth: in vitro, QSAR, and in vivo investigations. J Med Chem. 2005 May 5;48(9):3130-40. doi: 10.1021/jm040132t. PMID:15857119 doi:http://dx.doi.org/10.1021/jm040132t
- ↑ Martin MB, Grimley JS, Lewis JC, Heath HT 3rd, Bailey BN, Kendrick H, Yardley V, Caldera A, Lira R, Urbina JA, Moreno SN, Docampo R, Croft SL, Oldfield E. Bisphosphonates inhibit the growth of Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondii, and Plasmodium falciparum: a potential route to chemotherapy. J Med Chem. 2001 Mar 15;44(6):909-16. doi: 10.1021/jm0002578. PMID:11300872 doi:http://dx.doi.org/10.1021/jm0002578
- ↑ Martin MB, Sanders JM, Kendrick H, de Luca-Fradley K, Lewis JC, Grimley JS, Van Brussel EM, Olsen JR, Meints GA, Burzynska A, Kafarski P, Croft SL, Oldfield E. Activity of bisphosphonates against Trypanosoma brucei rhodesiense. J Med Chem. 2002 Jul 4;45(14):2904-14. doi: 10.1021/jm0102809. PMID:12086478 doi:http://dx.doi.org/10.1021/jm0102809
- ↑ Yang G, Zhu W, Kim K, Byun SY, Choi G, Wang K, Cha JS, Cho HS, Oldfield E, No JH. In Vitro and In Vivo Investigation of the Inhibition of Trypanosoma brucei Cell Growth by Lipophilic Bisphosphonates. Antimicrob Agents Chemother. 2015 Dec;59(12):7530-9. doi: 10.1128/AAC.01873-15., Epub 2015 Sep 21. PMID:26392508 doi:http://dx.doi.org/10.1128/AAC.01873-15
- ↑ Aripirala S, Gonzalez-Pacanowska D, Oldfield E, Kaiser M, Amzel LM, Gabelli SB. Structural and thermodynamic basis of the inhibition of Leishmania major farnesyl diphosphate synthase by nitrogen-containing bisphosphonates. Acta Crystallogr D Biol Crystallogr. 2014 Mar;70(Pt 3):802-10. doi:, 10.1107/S1399004713033221. Epub 2014 Feb 22. PMID:24598749 doi:http://dx.doi.org/10.1107/S1399004713033221
- ↑ Maheshwari S, Kim YS, Aripirala S, Murphy M, Amzel LM, Gabelli SB. Identifying Structural Determinants of Product Specificity in Leishmania major Farnesyl Diphosphate Synthase. Biochemistry. 2020 Jul 12. doi: 10.1021/acs.biochem.0c00432. PMID:32584028 doi:http://dx.doi.org/10.1021/acs.biochem.0c00432
- ↑ Zhang Y, Cao R, Yin F, Hudock MP, Guo RT, Krysiak K, Mukherjee S, Gao YG, Robinson H, Song Y, No JH, Bergan K, Leon A, Cass L, Goddard A, Chang TK, Lin FY, Van Beek E, Papapoulos S, Wang AH, Kubo T, Ochi M, Mukkamala D, Oldfield E. Lipophilic bisphosphonates as dual farnesyl/geranylgeranyl diphosphate synthase inhibitors: an X-ray and NMR investigation. J Am Chem Soc. 2009 Apr 15;131(14):5153-62. PMID:19309137 doi:10.1021/ja808285e
- ↑ Das S, Edwards PA, Crockett JC, Rogers MJ. Upregulation of endogenous farnesyl diphosphate synthase overcomes the inhibitory effect of bisphosphonate on protein prenylation in Hela cells. Biochim Biophys Acta. 2014 Apr 4;1841(4):569-73. doi:, 10.1016/j.bbalip.2013.12.010. Epub 2013 Dec 22. PMID:24369118 doi:http://dx.doi.org/10.1016/j.bbalip.2013.12.010
- ↑ Selby P. Alendronate treatment for osteoporosis: a review of the clinical evidence. Osteoporos Int. 1996;6(6):419-26. doi: 10.1007/bf01629572. PMID:9116385 doi:http://dx.doi.org/10.1007/bf01629572
Proteopedia Page Contributors and Editors (what is this?)
Hannah Campbell, Michal Harel, Tihitina Y Aytenfisu, Alexander Berchansky, Joel L. Sussman, Sandra B. Gabelli