Forkhead Box Protein 3 (
FOXP3) is a member of the
Forkhead transcription factor family. It is highly expressed in regulatory T (Treg) cells, a subset of CD4
+ T cells that play a critical role in suppressing immune responses, especially those mediated by autoreactive T cells.
[1] FOXP3 upregulates a number of genes like Cd25 and Ctla4 and represses other genes like
IL-2 and Ptpn22.
[2] As with many transcription factors, it cooperates with a number of transcription factor partners to regulate gene expression, including NFAT1, which participates in the inducible expression of cytokine genes like IL-2,
IL-4, and TNFα in T cells.
[3] A number of mutations to FOXP3 are known to result in a severe autoimmune disease known as IPEX (immune dysregulation, polyendocriopthy, enteropathy, X-linked). As FOXP3 is found on the X-chromosome, mutations to FOXP3 typically only display deleterious phenotypic traits in males, resulting in lymphocyte infiltration and wide spread inflammation in inphants.
[4] A similar pathology is also found in mice who carry nonsense mutations in the FOXP3 locus. These mutant mice are known as
scurfy mice. The targeted elimination of FOXP3
+ CD4
+ Tregs in adult mice has similar autoimmune dysfunction.
[5] Further, ectopic expression of FOXP3 in peripheral CD4
+CD25
- T cells equips these T cells with the ability to suppress the proliferation and effector functions of autoreactive T cells
in vivo.
[6]
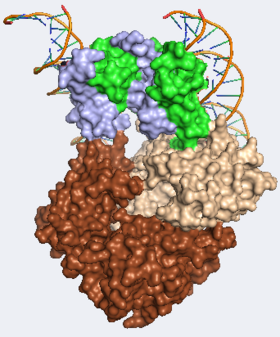
The interaction of FOXP3 with NFAT1 and the FOXP3-NFAT1 target sequences found in IL-2 has been investigated extensively. The appears to form a with a and two unique DNA , each containing distinct FOXP sites.[7]
Each domain-swapped dimer of FOXP3 makes extensive interactions with NFAT1 involving FOXP3 Thr359, Asn361, His365, while Glu399 and Glu401 of FOXP3 including Lys664, Arg665, Lys666, and Arg667, among others, which were critical in the FOXP2-NFAT1 interaction. These interactions allow FOXP3 and NFAT1 to bind more tightly together than other NFAT1 complexes formed with other Forkhead box proteins.[7] The utilize their DNA binding helices to bind unique sequences within the IL-2 promoter. These helices fit within the major groove of the IL-2 promoter ( and ), primarily using FOXP3 residues . These oligonucletodies are held in an antiparllel conformation, making FOXP3 unable to bind nearby FOXP3 binding sites, due to steric hindrance.[7]
The FOXP3 forkhead domain forms a relatively unique that bridges two unique oligonucletodies. This dimer is stabilized by a network of (Phe340, Leu345, Trp348, Trp366, and Met370) and , (Tyr364, Trp366, Phe371, Phe 373, and Trp381) all of which are highly conserved across the FOX superfamily. Mutations to several of these residues such as are known to occur in IPEX patients. Phe373 is of the dimer interface and the F373A mutation disrupts dimer formation. The F371C mutation does not appear to disrupt dimerization, probably because the aromatic ring of the phenylalanine residue is and thus probably does not play a critical role in dimer formation, but rather might disrupt overall FOXP3 function. Dimerization is unique to FOXP3 among the FOX superfamily likely due to residues . When these residues are mutated to Gln and Thr respectively, to match those residues found in FOXP2, dimer formation is abolished.[7] Here is a morph estimating the .
Clues toward the biological mechanism of action as to how mutation of dimer-stabilizing residues of FOXP3 causes IPEX can be garnered from microarray studies, which revealed a number of improperly regulated FOXP3 targets such as IL-2 and Ptpn22, and from in vitro suppression assays, which revealed that a number of dimer-destabilzing mutations eliminated the suppresive capacity of FOXP3+ cells.[7] These findings are consistent with clinical data, such as an infant bearing the F373A mutation developing autoimmune insulin-dependent diabetes within two weeks of life.[8] It is clear however from microarray data that not all known FOXP3 targets are impacted by FOXP3 dimer-disrupting mutations, indicating FOXP3 might form varied complexes depending upon the target it binds.[7]