G09SecL04Tpc2
From Proteopedia
|
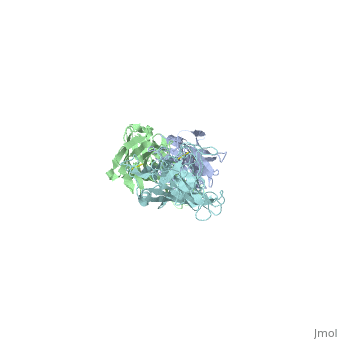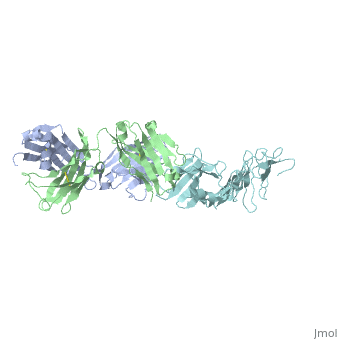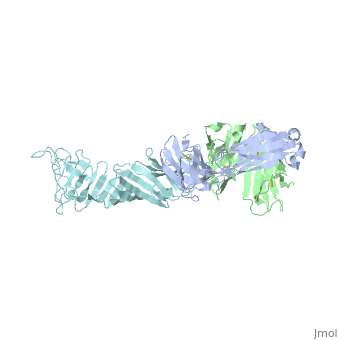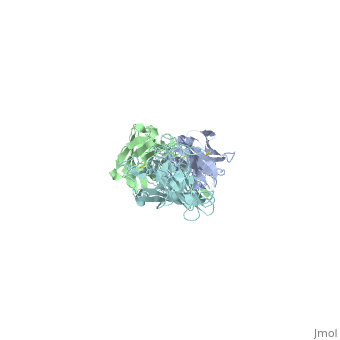
Outer Surface Protein A (OspA) is a major lipoprotein on the borrelia burgdorferi spirochete, which is a causative agent of Lyme Disease. The three loops in the C-terminus define the dimerized antigen-antibody complex. Shown to the right is the . The interaction between the loops and the LA-2 antibody forms the basis for successful OspA vaccination of this infectious disease. Most early symptoms include fever and headaches that are often eliminated through antibiotics. On the other hand, OspA has many important functions that are associated with late stage neurological disorders of the central nervous system including acute Lyme Neuroborreliosis, which can be extremely difficult to treat and disabling of the physical body itself.[1]
Contents |
OspA's Role in Lyme Disease
Lyme disease is the most common tick-borne disease in North American hemisphere. Responsible for transmission is a tick vector from the genus of Ixodidae. There are different bacterial strains of borrelia including B.afzellii and B.garinii being very prevalent throughout the European continent; other known strains such as B.dutonii and B. recurrentis have been discovered within the past few decades. The most common strain in the United States is borrelia burgdorgeri sensu stricto, and this treponema-like spirochete was discovered to be the causative agent of Lyme Disease discovered by Dr. Willy Burgdorferi in 1982 along with several other colleagues.[2]
Besides OspA, there are other outer surface proteins or lipoproteins, including OspB, OspC, and Vls (see 3-D Structures below), which are very important for the transmission of Lyme Disease from the tick to the host, as well as establishing the infection via bloodstream disseminating throughout the host body. Early symptoms of Lyme Disease include skin lesions and rashes that have a characteristic bulls-eye appearance known as erythema chronicum migrans (ECM). It is important to note that ECM can be used for early diagnosis of Lyme Disease prior to awaiting more accurate laboratory tests.[3]
Outer Surface Protein A (OspA)
Structural Breakdown
|
Outer Surface Protein A has approximately 270 amino acid residues.[4] In terms of , OspA is made up of 21 anti-parallel single layer beta strands (gold) and one alpha helix (magenta).[4] OspA has three major components to its structure: an , central sheet, and a c-terminal barrel domain. The n-terminal is often termed as a sandwich due to its jumbled formation of amino acid residues. Most of the beta strands lie on the . Lastly, the is nicknamed barrel domains because the three primary loops on the tip of the c-terminus connect to both sides of the central beta sheet forming a barrel or a hole in the middle. This can be seen by rotating the molecule.
The c-terminal is heavily involved in the antigen:antibody complex unlike the n-terminal. The c-terminal houses a protruding ridge of three loops: contains residues 203-220; contains residues 224-233; contains residues 246-257. These define the LA-2 (antibody) epitope discussed below. More specifically, the loops indicate where the antibody binds to the antigen. This protruding ridge of the c-terminus coupled with OspA’s elongated fold along the central beta sheet gives OspA a high degree of surface exposure and high antigenicity.
Scene List:
LA-2 Recognition
|
Since NMR chemical shifts are greatly sensitive to magnetic (local) environments, they are great for sensing conformational changes; NMR chemical-shift perturbation was an important method in identifying this three- loop epitope of OspA, but more importantly it also portrayed that these three loops were strongly antigenic.[4] In a study conducted in 2000, Wei Ding and fellow colleagues illustrated the through NMR and crystallography: LA-2 makes a concave-like groove over the 3 loops on the tip of the c-terminus through direct contact by heavy and light chains includng interactions between its R groups.[4]
Subsequent NMR results suggested that the overall c-terminal was basically unchanged by LA-2 binding, except for the minor shifts in the conformations of the 3 loops; the conformations result in order to accommodate interactions with the Fab.[4] Further analysis of amide proton exchange rates supported this by proving that the n-terminal and central sheet domains were stable while the c-terminal domain illustrated great fluctuation in respect to its 3 loops.[4] Specifically, Loops 2 and 3 were only subject to slight change in comparison to Loop 1’s dramatic increase.
Loop 1 protrudes the most along the C-terminal barrel domain probably because it has extra amino acid residues. More importantly, since it has a greater degree of protrusion, it has greater surface exposure making it particularly antigenic. Further evidence through Western blotting methods suggests that Loop 1 plays the greatest role in allowing LA-recognition of OspA.[4]
By understanding the binding site between OspA’s three loops and LA-2, greater research has been done in vaccinations against OspA. It is important to note that the vaccination is virtually ineffective once transmission into the host is complete mainly due to OspA’s down-regulation and hiding maneuvers. LA-2 antibody is a major proponent for effectiveness of the current OspA vaccine, but its effectiveness depends on different strains of borrelia. LA-2 recognizes the epitope of OspA from B.burgdorferi, but does recognize European strains including B.afzelli and B.garinii. This is due to sequence variation. is a great example. In B.afzelli and B.garinii there is a Glutamine (Q) at position 208. Several other variations are seen at Positions 215 and 251. At 215, B.garinii has a lysine group while B. burgdorferi and B.afzelli has an alanine group. At 251, B.burgdorferi has an Asparagine group but B.afzelli and B.garinii has an Alanine group. These sequence variations suggest that a multivalent OspA vaccine with a few strains will be more protective against infection of a range of Borrelia species.[4]
Pathogenesis of Lyme Neuroborreliosis (LNB)
Penetrating the immune system’s first line of defense the skin, Borrelia can cause a local infection at the site of tick attachment. These infections produce the easily recognizable bulls-eye rash associated with Lyme disease. While other antigens may play a greater role in the beginning of the invasion, OspA has been shown to be more active in the second stage of the infection after the bacteria spreads throughout the body. Affected areas can include the heart, joints, and the nervous system itself. The second stage is when signs of acute Lyme neuroborreliosis (LNB) begin. Symptoms of LNB include inflammation of the meninges and spinal nerve roots, radicular pain (Bannwarth’s syndrome), lymphocytic meningitis, in addition to cranial or peripheral neuritis.[5]
The Elusiveness of Borrelia
The Borrelia have developed very effective ways to prevent contact with the host’s immune system. This makes it difficult for the body to attack because of the elusive characteristics of the following mechanisms:
Down regulation of immunogenic surface proteins
Borrelia use antigenic variation to hide highly immunogenic surface proteins such as OspA and OspC. OspA is a potent stimulator for neutrophils and is known to induce a proinflammatory response. In order to not be detected by the immune system and not create an inflammatory response, OspA is generally down regulated in the beginning and not expressed in the early stages of Lyme disease.[5] It is very rare to detect any presence of OspA in the first six months of infection, however it is proposed that it forms earlier but remains in low levels or bound to immune complexes during this stage.[6]
Inactivation of effector mechanisms
Besides extensive down-regulation upon infection of the host, b.burgdorferi can make it even harder for the body to carry out a successful immune response. The borrelia can express complement-binding surface proteins, which prevents complement-mediated killing once inside the host regulated by the host’s immune system.[5] Although prior vaccination before infection is ideal, it is also important to develop and continue antibiotic research that can be administered after infection. In addition to eluding the immune system through these newly expressed surface proteins, borrelia also possesses the potential to activate anti-inflammatory cytokines such as Interleukin 10.[5] Ultimately, this suggests that borrelia can decrease inflammation by inducing IL-10 and preventing the host immune system from marking regions of antigenic activity. This can also delay or possibly halt the attack of leukocytes. Lastly, recent studies have shown that b.burgdorferi releases antigens that are soluble.[5] These antigens mingle with the antibodies that are specific for b.burgdorferi and prevent the antibodies from carrying out their role in defense. Not only does borrelia demonstrate abilities of suppressing the immune response, but also dramatically weakens the reliable effector mechanisms of the host immune system.
Hiding in the Extracellular Matrix
Spirochetes from Borrelia survive by hiding in less accessible regions such as the extracellular matrix. From here they are able to invade multiple organ sites and avoid being easily found. Leukocytes in the bloodstream are not able to detect the spirochetes in the extracellular matrix. Borrelia is able to bind to many proteins in the extracellular matrix ensuring the survival of its spirochetes. This is supported by the finding that spirochete associated plasmin degrades the protein fibronectin which is located on the extracellular matrix.[7]
Crossing the Blood-Brain Barrier to the CNS
After surviving the initial response from the immune system, the Borrelia makes its way to the central nervous system and crosses into the blood brain barrier. The fact that Borrelia enters the cerebrospinal fluid is well documented through PCR and culture methods, but its exact passing into the blood brain barrier is still at speculation. The two main theories involve transcellular passage and the penetration of the spirochetes between the endothelial cells or intercellular passage. The theory of paracellular passage also exists suggesting a combination of the two.[8] The brain microvascular endothelial cells, or BMECs, limit the transport of solutes into the brain and protects it from being invaded by pathogens. BMECs are connected by a web of tight intercellular junctions. Borrelia has been shown to bind to human BMEC at cell borders or near the tips supporting a paracellular transmigration theory. Activation cascades across the BMEC may result in the degradation of the tight cellular junctions at specific focal points resulting in a Borrelia invasion.[8]
3-D Structures
Additional Resources
References
- ↑ Cairns V, Godwin J. Post-Lyme borreliosis syndrome: a meta-analysis of reported symptoms. Int J Epidemiol. 2005 Dec;34(6):1340-5. Epub 2005 Jul 22. PMID:16040645 doi:10.1093/ije/dyi129
- ↑ Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317-9. PMID:7043737
- ↑ Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006 Nov 1;43(9):1089-134. Epub 2006 Oct 2. PMID:17029130 doi:10.1086/508667
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Ding W, Huang X, Yang X, Dunn JJ, Luft BJ, Koide S, Lawson CL. Structural identification of a key protective B-cell epitope in Lyme disease antigen OspA. J Mol Biol. 2000 Oct 6;302(5):1153-64. PMID:11183781 doi:10.1006/jmbi.2000.4119
- ↑ 5.0 5.1 5.2 5.3 5.4 Rupprecht TA, Koedel U, Fingerle V, Pfister HW. The pathogenesis of lyme neuroborreliosis: from infection to inflammation. Mol Med. 2008 Mar-Apr;14(3-4):205-12. PMID:18097481 doi:10.2119/2007-00091.Rupprecht
- ↑ Schutzer SE, Coyle PK, Dunn JJ, Luft BJ, Brunner M. Early and specific antibody response to OspA in Lyme Disease. J Clin Invest. 1994 Jul;94(1):454-7. PMID:8040289 doi:http://dx.doi.org/10.1172/JCI117346
- ↑ Fuchs H, Wallich R, Simon MM, Kramer MD. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12594-8. PMID:7809084
- ↑ 8.0 8.1 Grab DJ, Perides G, Dumler JS, Kim KJ, Park J, Kim YV, Nikolskaia O, Choi KS, Stins MF, Kim KS. Borrelia burgdorferi, host-derived proteases, and the blood-brain barrier. Infect Immun. 2005 Feb;73(2):1014-22. PMID:15664945 doi:10.1128/IAI.73.2.1014-1022.2005