From Proteopedia
proteopedia linkproteopedia link OspA + LA-2 Complex
Background
Lyme disease, discovered in 1975[1], is a vector--borne disease caused by the inoculation of a spirochaete, specifically Borellia burgdorferi, into the skin by members of hard bodied ticks of the family Ixodes. Symptoms include arthritis at major joints, neurological problems such as reduced memory and poor ability to concentrate, and characteristic lesions erythema migans, more commonly known as the bull’s eye rash. A major outer surface membrane protein of Borrelia, ospA, plays a key role in immunity against Lyme disease through its binding to the LA-2 fab. The Fragment Antigen Binding (fab) consists of a heavy chain and light chain, with each respective region having its own constant and variable regions.

Using darkfield microscopy technique, this photomicrograph, magnified 400x, reveals the presence of spirochaete, or "corkscrew-shaped" bacteria known as Borrelia burgdorferi
Introduction to LYMErix
LYMErix, implemented in 1998 by the FDA,is a noninfectious vaccine that uses recombinant ospA expressed by Escherichia coli absorbed into an aluminum hydroxide adjuvant to stimulate immune response during injection. The formation of specific IgG anti- ospA antibodies including those directed towards the epitope LA-2 [2]that resulted following vaccination aimed at traveling to the tick’s mid-gut in order to interact with B.burgdorferi, preventing transmission of Lyme disease to host.
Vaccine efficiency was evaluated by immunizing mice with LYMErix and using ELISA to measure their serum antibody response to ospA. In 1996, clinical trials conducted in highly endemic areas of the United States with sample size exceeding 10,000 subjects, LYMErix was found to confer protective immunity to B. burgdorferi in 76% of adults and 100% of children with only mild and short term effects[2].
Development of the recombinant vaccine
Initial approaches for vaccines to treat lyme disease solely focused on ospA after studies involving passive immunization in mice. Studies indicated that passive immunization in mice only occurred if the blood from humans infected with Lyme disease contained anti-ospA monoclonal antibodies[3] . Although ospA vaccines, such as Lymerix, were able to halt transmission of the spirochetes, they were immediately pulled from the market due to chronic side effects such as severe arthritis. Additionally, quantitative analysis of immune response[3] of the whole ospA indicated that it is not predictive of protection.
Recent developments in vaccines against Lyme Borreliosis emphasize the need to utilize the LA-2 –ospA complex on the basis that it, as a result of its specificity, induces long- term immune response in mice during previously studied clinical trials. Passive immunization of mice that resulted with significant titers of LA-2 serum antibody had a strong correlation with protection against tick transmission of infection[4]. The LA-2 ospA complex constitutes the interaction between Borrelia membrane ospA and LA-2 fab, an epitope of the ospA associated with protective immunity after vaccination.[2]
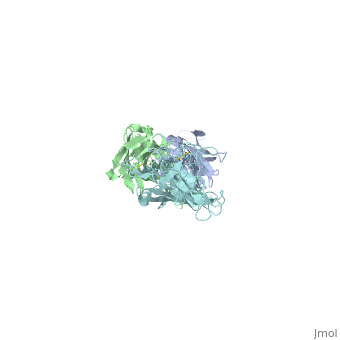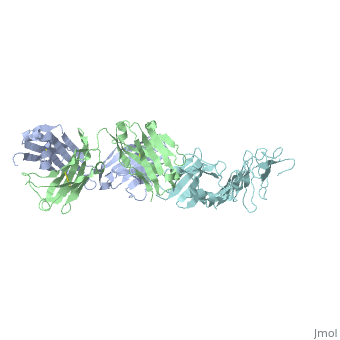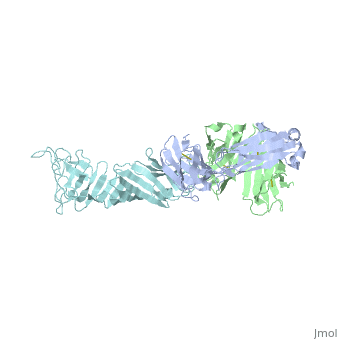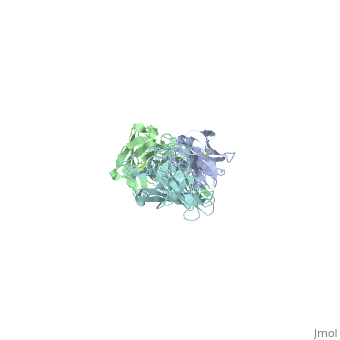
Structure of LA-2 OspA complex
| ---
LA-2 fab recognizes the three surface-exposed loops; loop1, loop 2 and loop 3 of the C-terminal domain of OspA that are on the tip of the elongated molecule most distant from the lipid-modified N terminus. Residues 203 to 220 in “loop 1“,residues 224 to 233 in “loop 2“ and residues 246 to 257 in “loop 3“are mostly effected by la-2 binding. In loop 1 residues 206 and 216 are not affected. The interactions between OspA and LA-2 include eight direct hydrogen bonds, four solvent-bridged hydrogen bonds, three ion pairs, and numerous van der Waals interactions.LA-2 recognition of OspA involves an induced fit mechanism where loop 1-3 conformations shift to optimize complementarity to the antigen-combining site. [5]
Limitations
Although the LA-2- ospA complex induces a high reactivity of protective immune response for B. burgdorferi, it lacks the broad specificity to encompass other genospecies of Borrelia. In North America, Lyme borreliosis is caused by a single strain B.burgdorferi, whereas in parts of Europe and Asia, a minimum of three strains, B. afzelli, B. burgdorferi, and B. garinii are involved in infecting humans[6]. The lack of broad specificity in the LA-2- OspA complex is due to the presence of highly variable regions in ospA.
------
Trp-216
One hyper-variable region is the Tryptophan amino acid residue sequence 216. Polar amino acid side chains flank the Tryptophan, and changes in such amino acids can ultimately affect the reactivity of OspA to monoclonal antibodies[7]. Differences in side chains of amino acids on the polar surface of a helical structure surrounding the highly conserved tryptophan residue 216 can affect the reactivity of OspA.
Ala-208
In B. burgdorferi, residue 208 expresses Alanine, whereas in B. afzelii, and B. garinii, Glutamine is expressed. The variation in this "first loop" residue is another factor, which inhibits successful antibody cross-reactivity between other strains of Borrelia. LA-2 and OspA of B. burgdorferi forms a tight border when binding and this is promoted by the Alanine 208 residue. The Glutamine residue that is found in B. afzelii and B. garinii is longer and more problematic when it comes to binding[5]. It creates a looser, more ineffective interface between the LA-2 and OspA, which conclusively makes an ineffectual vaccine.
Residues 217-237
This region is crucial in determining the effectiveness of the vaccine. The binding of OspA and the LA-2 FAB is defined by the amino acid residue sequence 217-237, and causes nonspecific binding thus inhibiting the LA2-FAB-OspA complex binding to other species of Borrelia[8].
Literature References
- ↑ Steere AC. Lyme borreliosis in 2005, 30 years after initial observations in Lyme Connecticut. Wien Klin Wochenschr. 2006 Nov;118(21-22):625-33. PMID:17160599 doi:10.1007/s00508-006-0687-x
- ↑ 2.0 2.1 2.2 Poland GA. Vaccines against Lyme disease: What happened and what lessons can we learn? Clin Infect Dis. 2011 Feb;52 Suppl 3:s253-8. doi: 10.1093/cid/ciq116. PMID:21217172 doi:10.1093/cid/ciq116
- ↑ 3.0 3.1 Luft BJ, Dunn JJ, Lawson CL. Approaches toward the directed design of a vaccine against Borrelia burgdorferi. J Infect Dis. 2002 Feb 15;185 Suppl 1:S46-51. PMID:11865439 doi:10.1086/338463
- ↑ Embers ME, Narasimhan S. Vaccination against Lyme disease: past, present, and future. Front Cell Infect Microbiol. 2013;3:6. doi: 10.3389/fcimb.2013.00006. Epub 2013, Feb 12. PMID:23407755 doi:10.3389/fcimb.2013.00006
- ↑ 5.0 5.1 •Ding W, Huang X, Yang X, Dunn JJ, Luft BJ, Koide S, Lawson CL. Structural identification of a key protective B-cell epitope in Lyme disease antigen OspA. J Mol Biol. 2000 Oct 6;302(5):1153-64.PMID:11183781 doi:10.1006/jmbi.2000.4119
- ↑ Livey I, O'Rourke M, Traweger A, Savidis-Dacho H, Crowe BA, Barrett PN, Yang X, Dunn JJ, Luft BJ. A new approach to a Lyme disease vaccine. Clin Infect Dis. 2011 Feb;52 Suppl 3:s266-70. PMID:21217174 doi:10.1093/cid/ciq118
- ↑ McGrath BC, Dunn JJ, Gorgone G, Guttman D, Dykhuizen D, Luft BJ. Identification of an immunologically important hypervariable domain of major outer surface protein A of Borrelia burgdorferi. Infect Immun. 1995 Apr;63(4):1356-61. PMID:7890394
- ↑ Connolly SE, Benach JL. The versatile roles of antibodies in Borrelia infections. Nat Rev Microbiol. 2005 May;3(5):411-20. PMID:15864264 doi:10.1038/nrmicro1149
Notes
|