Function
Glycoprotein Gp120, named for its molecular weight, is found on the surface of HIV envelope. Gp120 is associated with Gp41. It is involved in the binding of HIV to CD4 receptors thus attaching the virus to the host cell[1]. For more details see
Structural highlights
In HIV allows the virus to infect human immune cells.
Molecular Mechanism of HIV-1 gp120 Mutations That Reduce CD4 Binding Affinity [2]
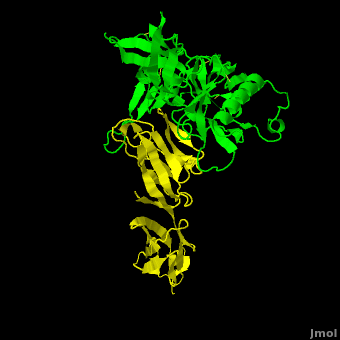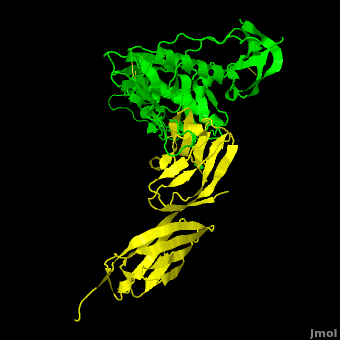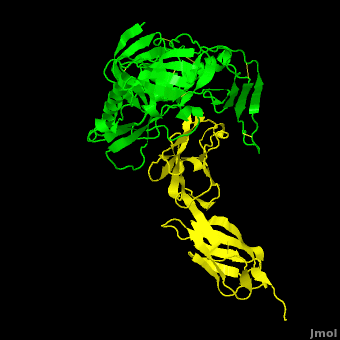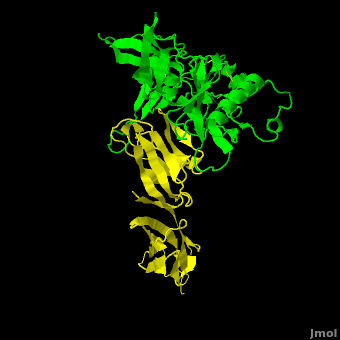
Despite abundant efforts in HIV research the HI-virus remains one of the most serious infectious health threats for human worldwide. Infection involves the entry of the virus into CD4 carrying cells and is mediated by the recognition of the (colored in yellow) by (colored in green). The CD4-binding site of gp120 contains , termed the ‘CD4-binding loop’ (in blue) and the ‘outer domain exit (ODE) loop’ (colored in salmon), respectively. Importantly, is a key target site for gp120-ligands to interfere with the gp120-CD4 interaction and thereby hampering infection. Carbons of the important residues are colored in white, nitrogens in blue, and oxygens in red. Therapeutic ligands include for instance soluble CD4 and its variants.
For the development of more potent gp120-ligands targeting the CD4-binding site we investigated two gp120-proteins from two clinical HIV isolates that exhibit a decreased affinity for soluble CD4. Each variant exhibits a set of simultaneous mutations in the CD4-binding loop and the ODE-loop. The exhibits three mutations in the CD4-binding loop (P369A, I371L, T373M) and two mutations in the ODE-loop (G471E, D474N). The protein contains three simultaneous mutations: V372E, T373M in the CD4-binding loop, and D474N in the ODE-loop. Carbons of the mutated residues are colored in white, nitrogens in blue, oxygens in red, and sulfur in yellow. To understand the phenomenon of reduced CD4-binding on a structural level the two gp120 variants were modeled, each with its specific set of mutations, and simulated using molecular dynamics. The results show that the mutant glutamates of both variants play a key role for reduced CD4 binding by affecting the conformation of the CD4-binding and ODE-loop. This knowledge should be helpful to predict the resistance of novel gp120 mutants or to design gp120-ligands with improved binding properties.
3D structures of Gp120
Gp120 3D structures