Group:MUZIC:Mena VASP
From Proteopedia

Contents |
Mena/Ena/VASP
Introduction
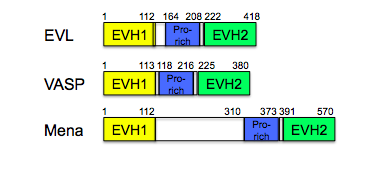
The Mena/Ena/VASP protein family contributes to the cell motility and cell adhesion through the regulation of actin dynamics. The family includes three members in mammals: Vasodilator-stimulated phosphoprotein (VASP), Mena (mammalian enabled), and the Ena-VASP-like protein. The Mena/Ena/VASP proteins commonly feature similar domain composition consisting of an N-terminal Ena/Vasp like homology domain 1 (EVH1), a central proline-rich region and a C-terminal Ena/Vasp like homology domain 2 (EVH2). Mena/VASP proteins associate with actin filaments and their complexes reside in the hot spots of actin reassembly, such as tips of lamellipodia and filopodia.
Sequence annotation
Mena/Ena/VASP proteins differ in the size of proline-rich region and Mena contains a 91-residue region between the EVH1 domain and proline-rich core, with 13 repeats of a 5-residue “LERER" motif [1].
Mena (Protein enabled homolog) (uniprot:Q8N8S7)
Ena (uniprot:Q9UI08)
VASP (uniprot:P50552)
Structure
|
|
Currently available structural information on the EVH1 and the coil-coiled part of EVH2 domains comprises specifically:
i) The NMR Structure of EVH1 domain of human VASP. PDB Code:1EGX
ii) 1usd 1use Crystal structure of C-terminal coil -coiled part of EVH2 domain of human VASP. PDB Code:1USD, PDB Code:1USE
iii) 1evh Crystal structure of EVH1 domain of Mena (Mus Musculus) in complex with FPPP peptide. PDB Code:1EVH
Function and interaction
The EVH1 domain of Mena/Ena/VASP proteins possesses specific affinity towards various cytoskeleton proteins containing FPPPP motif, such as vinculin [2], lamellipodin [3], zyxin [4], palladin [5] and Xin [6]. Additionally, the Pro-rich region of Mena/Ena/VASP associates with profilin and the SH3 and WW domains of various signaling and scaffolding proteins. The EVH2 domain features three highly conserved motifs: the F- and G-Actin binding sites and the C-terminal right-handed coiled coil region, which is also known as tetramerization domain [7]. The tetramer is formed through parallel orientation of the C-terminal ends and its structure is stabilized by tight hydrophobic packing and a network of salt bridges [8]. Such oligomeric arrangement of Mena/VASP molecules provides an increased concentration of different ligands, as well as G-actin in close vicinity to growing actin filaments. It is suggested that Mena/VASP proteins bind to the barbed end of actin filaments and prevent their capping by CapZ [9]. Mena/Ena/VASP proteins also contribute to the nucleation and elongation steps of actin filaments formation. It is shown that the deficiency of Mena/Ena/VASP in lamellipodia leads to formation of abnormally short and highly branched filaments. In contrast, their excess resulted in the appearance of longer, sparsely branched structures [10].
It was demonstrated recently that Mena interacts with fibronectin receptor α5β1 integrin via the “LERER" motif [11]. The regulation of VASP protein activity occurs through phosphorylation of Ser-157, Ser-239, and Thr-278 by cAMP-and cGMP-dependent protein kinases (PCA/PCG) via unknown mechanisms. Interestingly, the first two phosphorylation sites are conserved in Mena, whereas the Ena member contains only the first one [12] [13].
Pathology
It was shown that delocalization of Mena/Ena/VASP proteins from intercalated discs at the interface of myocytes causes severe cardiomyopathy [14]. The Mena gene can be alternatively spliced and different Mena isoforms manifest themselves during tumor progression. In particular, an exon encoding a sequence of 19 amino acids inserted between the EVH1domain and the LERER repeats generates so called Mena invasion isoform. Translation of another exon which encodes a 21 amino acid insertion in the EVH2 domain produces the Mena11a isoform [15][16]. The expression of Mena invasion isoform is increased in invasive tumor cells, the amount of Mena 11a isoform being comparatively lower [17]. The Mena protein has been successfully used as a component of marker pattern for the assessment of metastatic risk called Tumor Micro-Environment of Metastasis (TMEM) [18].
References
- ↑ Gertler FB, Niebuhr K, Reinhard M, Wehland J, Soriano P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell. 1996 Oct 18;87(2):227-39. PMID:8861907
- ↑ Reinhard M, Rudiger M, Jockusch BM, Walter U. VASP interaction with vinculin: a recurring theme of interactions with proline-rich motifs. FEBS Lett. 1996 Dec 9;399(1-2):103-7. PMID:8980130
- ↑ Krause M, Leslie JD, Stewart M, Lafuente EM, Valderrama F, Jagannathan R, Strasser GA, Rubinson DA, Liu H, Way M, Yaffe MB, Boussiotis VA, Gertler FB. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev Cell. 2004 Oct;7(4):571-83. PMID:15469845 doi:S1534580704003302
- ↑ Drees B, Friederich E, Fradelizi J, Louvard D, Beckerle MC, Golsteyn RM. Characterization of the interaction between zyxin and members of the Ena/vasodilator-stimulated phosphoprotein family of proteins. J Biol Chem. 2000 Jul 21;275(29):22503-11. PMID:10801818 doi:10.1074/jbc.M001698200
- ↑ Boukhelifa M, Parast MM, Bear JE, Gertler FB, Otey CA. Palladin is a novel binding partner for Ena/VASP family members. Cell Motil Cytoskeleton. 2004 May;58(1):17-29. PMID:14983521 doi:10.1002/cm.10173
- ↑ van der Ven PF, Ehler E, Vakeel P, Eulitz S, Schenk JA, Milting H, Micheel B, Furst DO. Unusual splicing events result in distinct Xin isoforms that associate differentially with filamin c and Mena/VASP. Exp Cell Res. 2006 Jul 1;312(11):2154-67. Epub 2006 Apr 24. PMID:16631741 doi:S0014-4827(06)00110-8
- ↑ Bachmann C, Fischer L, Walter U, Reinhard M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem. 1999 Aug 13;274(33):23549-57. PMID:10438535
- ↑ Kuhnel K, Jarchau T, Wolf E, Schlichting I, Walter U, Wittinghofer A, Strelkov SV. The VASP tetramerization domain is a right-handed coiled coil based on a 15-residue repeat. Proc Natl Acad Sci U S A. 2004 Dec 7;101(49):17027-32. Epub 2004 Nov 29. PMID:15569942
- ↑ Bachmann C, Fischer L, Walter U, Reinhard M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem. 1999 Aug 13;274(33):23549-57. PMID:10438535
- ↑ Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, Gertler FB. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002 May 17;109(4):509-21. PMID:12086607
- ↑ Gupton SL, Riquelme D, Hughes-Alford SK, Tadros J, Rudina SS, O Hynes R, Lauffenburger D, Gertler FB. Mena binds alpha5 integrin directly and modulates alpha5beta1 function. J Cell Biol. 2012 Aug 20;198(4):657-76. PMID:22908313 doi:10.1083/jcb.201202079
- ↑ Benz PM, Blume C, Seifert S, Wilhelm S, Waschke J, Schuh K, Gertler F, Munzel T, Renne T. Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J Cell Sci. 2009 Nov 1;122(Pt 21):3954-65. Epub 2009 Oct 13. PMID:19825941 doi:10.1242/jcs.044537
- ↑ Blume C, Benz PM, Walter U, Ha J, Kemp BE, Renne T. AMP-activated protein kinase impairs endothelial actin cytoskeleton assembly by phosphorylating vasodilator-stimulated phosphoprotein. J Biol Chem. 2007 Feb 16;282(7):4601-12. Epub 2006 Nov 2. PMID:17082196 doi:10.1074/jbc.M608866200
- ↑ Eigenthaler M, Engelhardt S, Schinke B, Kobsar A, Schmitteckert E, Gambaryan S, Engelhardt CM, Krenn V, Eliava M, Jarchau T, Lohse MJ, Walter U, Hein L. Disruption of cardiac Ena-VASP protein localization in intercalated disks causes dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2003 Dec;285(6):H2471-81. Epub 2003 Aug, 21. PMID:12933343 doi:http://dx.doi.org/10.1152/ajpheart.00362.2003
- ↑ Roussos ET, Balsamo M, Alford SK, Wyckoff JB, Gligorijevic B, Wang Y, Pozzuto M, Stobezki R, Goswami S, Segall JE, Lauffenburger DA, Bresnick AR, Gertler FB, Condeelis JS. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J Cell Sci. 2011 Jul 1;124(Pt 13):2120-31. PMID:21670198 doi:10.1242/jcs.086231
- ↑ Di Modugno F, Iapicca P, Boudreau A, Mottolese M, Terrenato I, Perracchio L, Carstens RP, Santoni A, Bissell MJ, Nistico P. Splicing program of human MENA produces a previously undescribed isoform associated with invasive, mesenchymal-like breast tumors. Proc Natl Acad Sci U S A. 2012 Nov 20;109(47):19280-5. doi:, 10.1073/pnas.1214394109. Epub 2012 Nov 5. PMID:23129656 doi:10.1073/pnas.1214394109
- ↑ Roussos ET, Balsamo M, Alford SK, Wyckoff JB, Gligorijevic B, Wang Y, Pozzuto M, Stobezki R, Goswami S, Segall JE, Lauffenburger DA, Bresnick AR, Gertler FB, Condeelis JS. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J Cell Sci. 2011 Jul 1;124(Pt 13):2120-31. PMID:21670198 doi:10.1242/jcs.086231
- ↑ Robinson BD, Jones JG. Tumor microenvironment of metastasis (TMEM): a novel tissue-based assay for metastatic risk in breast cancer. Future Oncol. 2009 Sep;5(7):919-21. doi: 10.2217/fon.09.79. PMID:19792958 doi:10.2217/fon.09.79