Group:MUZIC:Nebulin
From Proteopedia

Contents |
Nebulin
Introduction
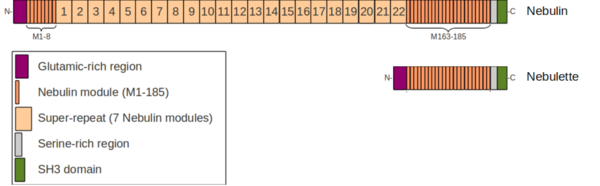
Nebulin (UniProt ID: P20929 [1]) is a very large filamentous protein (600-900 kDa) [1] tigthly associated to the thin filament of the muscle sarcomere throughout its length. Nebulin is mostly found within the sarcomeres of skeletal muscles but was also identified at a low level in cardiac muscle cells [2]. However, the sarcomeres of cardiac muscles predominantly contain a nebulin-like protein called nebulette (UniProt ID: O76041 [2]) which is a "short version" of nebulin (100 kDa), highly similar to its C-terminal part [3].
Sequence annotation
As illustrated on the schematic view, nebulin has a highly modular structure mostly consisting in the so-called nebulin modules. Nebulin modules are short sequences of 35 residues specific of the nebulin family of proteins. They are found in nebulin and nebulette, as well as in 3 other proteins characterized by the presence of a N-terminal LIM domain (N-RAP, Lasp-1 and Lasp-2). [4] Whereas Nebulette is a nearly integral Z-disk protein, nebulin extends further away towards the A-band. The minimal Z-disk region of nebulin has not been clearly defined yet, but it is likely to start around the module M170 up to the SH3 domain. Nebulin always includes the SH3 domain, and the Glutamic and Serine-rich regions, but differential splicing occurring within the nebulin modules region is the origin for a large variety of isoforms found at different developmental stages, and correlated to differences in sarcomere structure and properties, notably by affecting the Z-disk width. [5]
Structure
|
The SH3 domain is the only domain of nebulin for which a native 3D structure is available (PDB: 1ark). [6]
The nebulin SH3 domain adopts the typical of a SH3 domain: it consists in a β-Barrel made of 6 β-strands which are organised in 2 anti-parallel β-sheets.
Some NMR data obtained from nebulin modules in SDS have revealed the ability of the single modules to fold into a transient helix. They were proposed to undergo a folding transition upon binding to the central groove of a monomer of F-actin. [7]
Function and interactions
Function
The nebulin protein is involved in the structural integrity of the sarcomeres and is linked to many signalling pathways crucial for the maintenance of the sarcomere. The main functions of nebulin are [8]:
- To define and regulate the length of the thin filaments of actin (molecular ruler);
- To maintain the alignment of adjacent myofibrills (linker of adjacent Z-disks);
- To regulate muscle contraction (regulator of cross-bridge cycles).
Binding partners of the nebulin modules
- Tropomodulin: The N-terminal modules bind tropomodulin at the pointed end of the actin filament.
- Actin: Each nebulin module is able to bind to a monomer of F-actin. Even though the mechanism has not been clearly described yet, this interaction is thought to control the length of the thin filament.
- Tropomyosin/Troponin: The central super-repeats of 7 Neb modules bind 1 tropomyosin/troponin complex.
- Myosin: The central super-repeats were also shown to bind myosin in a way that would regulate the actomyosin activity.
- Calmodulin: The N-terminal and C-terminal super-repeats can bind calmodulin in a Calcium-independant manner.
- CapZ: The C-terminal modules can bind the barbed end capping protein CapZ.
- Desmin: The C-terminal modules located close to the Z-disk bind desmin, which is likely to play a role in the alignment of adjacent myofibrils.
- Alpha-Actinin: The C-terminal modules located within the Z-disk bind α-actinin
Binding partners of the Serine-rich region
The Ser-rich region has no homology to known structural motifs. The Serine residues can be phosphorylated by GSK3-β in a way that modulates some of the Nebulin interactions. [9]
Binding partners of the SH3 domain
The SH3 domain of nebulin usually interacts with the Proline-rich motif of its binding partners.
- Titin: Even though they are unlikely to be colocalised in vivo, the SH3 domain of nebulin has been proposed to bind to Pro-rich motifs located within the elastic PEVK region of titin. This has been shown in vitro. [10]
- Myopalladin: The SH3 domain also binds the central region of myopalladin, which allows its targeting to the Z-disk.[11] [10]
- N-WASP: The SH3 domain binds the N-WASP protein during early stages of myofibrillogenesis, this interaction promoting nucleation of the thin filament of actin. [12]
Pathology
Mutations in the nebulin encoding gene (NEB) are the most common cause of Nemaline myopathy (NM), a non-dystrophic congenital muscle disorder characterised by muscle weakness and hypotonia. At the cell level, the muscle fibers of patients contain rod-like “nemaline” bodies, composed of Z-disc and thin filament proteins. [13] Mutations are of different types, deletion, missenses, frameshifts, and have been found all along the sequence of nebulin and the disease phenotypes are rather similar whatever the type or location of the mutations. The mechanism of pathogenesis is not clear yet, but it has been proposed to be linked to the loss of nebulin isoforms, because of early truncation or loss of splicing sites, the subsequent l oss of fiber-type diversities necessary for proper muscle development. [14]
3D structures
References
- ↑ Wang K. Cytoskeletal matrix in striated muscle: the role of titin, nebulin and intermediate filaments. Adv Exp Med Biol. 1984;170:285-305. PMID:6547565
- ↑ Kazmierski ST, Antin PB, Witt CC, Huebner N, McElhinny AS, Labeit S, Gregorio CC. The complete mouse nebulin gene sequence and the identification of cardiac nebulin. J Mol Biol. 2003 May 9;328(4):835-46. PMID:12729758
- ↑ Moncman CL, Wang K. Nebulette: a 107 kD nebulin-like protein in cardiac muscle. Cell Motil Cytoskeleton. 1995;32(3):205-25. PMID:8581976 doi:http://dx.doi.org/10.1002/cm.970320305
- ↑ Pappas CT, Bliss KT, Zieseniss A, Gregorio CC. The Nebulin family: an actin support group. Trends Cell Biol. 2011 Jan;21(1):29-37. doi: 10.1016/j.tcb.2010.09.005. Epub 2010, Oct 15. PMID:20951588 doi:10.1016/j.tcb.2010.09.005
- ↑ Buck D, Hudson BD, Ottenheijm CA, Labeit S, Granzier H. Differential splicing of the large sarcomeric protein nebulin during skeletal muscle development. J Struct Biol. 2010 May;170(2):325-33. doi: 10.1016/j.jsb.2010.02.014. Epub 2010 , Feb 20. PMID:20176113 doi:10.1016/j.jsb.2010.02.014
- ↑ Politou AS, Millevoi S, Gautel M, Kolmerer B, Pastore A. SH3 in muscles: solution structure of the SH3 domain from nebulin. J Mol Biol. 1998 Feb 13;276(1):189-202. PMID:9514727 doi:10.1006/jmbi.1997.1521
- ↑ Pfuhl M, Winder SJ, Pastore A. Nebulin, a helical actin binding protein. EMBO J. 1994 Apr 15;13(8):1782-9. PMID:8168478
- ↑ Ottenheijm CA, Granzier H. Lifting the nebula: novel insights into skeletal muscle contractility. Physiology (Bethesda). 2010 Oct;25(5):304-10. PMID:20940435 doi:10.1152/physiol.00016.2010
- ↑ Takano K, Watanabe-Takano H, Suetsugu S, Kurita S, Tsujita K, Kimura S, Karatsu T, Takenawa T, Endo T. Nebulin and N-WASP cooperate to cause IGF-1-induced sarcomeric actin filament formation. Science. 2010 Dec 10;330(6010):1536-40. doi: 10.1126/science.1197767. PMID:21148390 doi:10.1126/science.1197767
- ↑ 10.0 10.1 Ma K, Wang K. Interaction of nebulin SH3 domain with titin PEVK and myopalladin: implications for the signaling and assembly role of titin and nebulin. FEBS Lett. 2002 Dec 18;532(3):273-8. PMID:12482578
- ↑ Bang ML, Mudry RE, McElhinny AS, Trombitas K, Geach AJ, Yamasaki R, Sorimachi H, Granzier H, Gregorio CC, Labeit S. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol. 2001 Apr 16;153(2):413-27. PMID:11309420
- ↑ Takano K, Watanabe-Takano H, Suetsugu S, Kurita S, Tsujita K, Kimura S, Karatsu T, Takenawa T, Endo T. Nebulin and N-WASP cooperate to cause IGF-1-induced sarcomeric actin filament formation. Science. 2010 Dec 10;330(6010):1536-40. doi: 10.1126/science.1197767. PMID:21148390 doi:10.1126/science.1197767
- ↑ Pelin K, Hilpela P, Donner K, Sewry C, Akkari PA, Wilton SD, Wattanasirichaigoon D, Bang ML, Centner T, Hanefeld F, Odent S, Fardeau M, Urtizberea JA, Muntoni F, Dubowitz V, Beggs AH, Laing NG, Labeit S, de la Chapelle A, Wallgren-Pettersson C. Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2305-10. PMID:10051637
- ↑ Pelin K, Donner K, Holmberg M, Jungbluth H, Muntoni F, Wallgren-Pettersson C. Nebulin mutations in autosomal recessive nemaline myopathy: an update. Neuromuscul Disord. 2002 Oct;12(7-8):680-6. PMID:12207938
Proteopedia Page Contributors and Editors (what is this?)
Marie-Cecile Pelissier, Michal Harel, Jaime Prilusky, Nikos Pinotsis