Group:MUZIC:Telethonin
From Proteopedia

Contents |
Telethonin
Also known as T-Cap or Titin Cap protein.
Introduction
Telethonin is a small protein composed of 167 amino acids with a molecular weight of 19KDa predominantly expressed in striated muscle. Located at the Z-disk, it is involved with the structural machinery of the sarcomere, linking titin and other proteins implicated in sarcomere structure and signalling pathways.
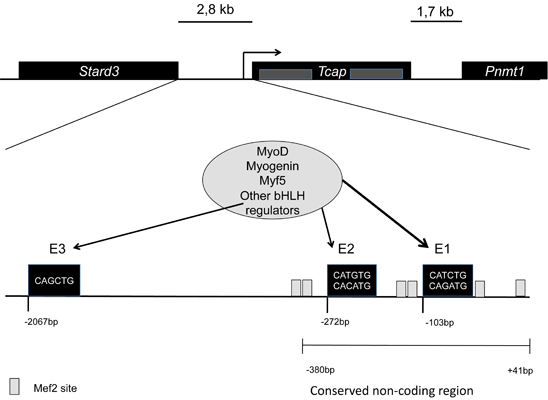
Telethonin is encoded by the TCAP in humans (Homo sapiens), located on the long arm of chromosome 17, and by Tcap gene in mice (Mus musculus) located on chromosome 11. No known homologues have been reported,Tcap is encoded by two exons, and has non-conserved intragenic sequences. The gene is flanked by two other genes: Stard3 upstream separated by 2,8kb, and Pnmt1 downstream separated by 1,7kb. Tcap has three conserved E-box elements at -103bp (E1), -272bp (E2), and -2067bp (E3). Transcriptional activation of Tcap depends predominantly on the regulation of E1, with MyoD playing an important role throughout development, and myogenin more important during late differentiation into myoblasts. [1]
Tcap is one of the most abundant transcripts in skeletal muscle [2] and is expreessed at similar levels in fast and slow skeletal muscle, althought expression levels are lower in neonatal compared to adult striated muscle. The transcript accumulates in a linear pattern similar to that of the myosin heavy chain [3]. Studies have reported that denervation leads to decrease expression of Tcap, suggesting that locomotor activity is a potential regulator .
|
Sequence Annotation
Telethonin is formed of 167 amino acids, and exhibits high similarity among species. The sequences of human, mouse, bovine, porcine telethonin are available from Uniprot.
Structure
Accumulation of telethonin is restricted to skeletal and cardiac, and t is one of the major components of the sarcomere. While predominantly localized at the Z-disk, it has also been reported to be localized within the nucleus.[4], [5]
Structural studies by Zou et al. [6] (N-terminal in blue and C-ter in orange). This structure is only found in the presence of , Telethonin might adopt a different fold in its absence.
The structure of telethonin was determined using X-ray crystallography. [7],[6] The shape and architecture of the complex of Titin/Telethonin was studied by small-angle- X-ray scattering (SAXS) and then compared to the crystallographic models. [8]
This symmetry of telethonin permits its interaction with titin. Both are assembled in an antiparallel (titin:telethonin). Titin N-terminal domains Z1 and Z2 (two Ig like repeats) interact with the C-terminal region of telethonin (residues 1-53), whereas telethonin mediates the antiparallel assembly of the two Z1Z2 domains.
Function and Interactions
In early differentiating myocytes titin C-terminal and telethonin co-localize, targeting titin kinase is close to C-terminal phosphorylation site of telethonin. This phosphorylation is involved in the reorganization of the cytoskeleton during myofibrillogenesis. [9] This particular co-localization is not seen in adult myofibrils, and titin kinase is instead localized at the M-band [9]; It has also been reported that telethonin interacts with other proteins including potassium channel β-subunit of the slow activating component of the delayed rectifier potassium current (IKs) channel (minK) [10], Ankyrin1 [11], and Z-disk proteins FATZ,/Myozenin-1/ Calsarcin-3 [12], and Ankrd2.[13]
Telethonin interacts with minK’s cytoplasmic domain, and minK binds specifically to the sixteen C-terminal residues of telethonin. This suggests that minK, telethonin and titin form a complex that links myofibrils to the sarcolemma. This process can be negatively regulated by the phosphorylation of telethonin on Ser157. This interaction has been shown to occur in cardiac myofibrils, but it has not been reported to exist in skeletal muscle, where minK is not expressed. [10].
Telethonin interacts with the N-terminus of FATZ/Myozenin-1/Calsarcin-2 between residues 78-125. This interaction may play a role in mechanosensation and stretch-associated signaling. [12] As part of the mechanical stress/stretch sensor machinery, telethonin interacts with Ankrd2, to transmit the signal to the nucleus so Ankrd2 can regulate gene expression. [13]
Telethonin is also involved in signalling processes that regulate muscle development. A feed back loop is formed with Myogenic Regulatory Factors (MyoD, myogenin, Myf5), telethonin and myostatin. MyoD possitively regulates telethonin, which then inhibits myostatin by direct interaction. While ‘’MyoD’’ expression is repressed by the myostatin-Smad3 pathway, repression of ‘’MyoD’’ itself is lost when myostatin is inhibited by telethonin. [14] [15]
Telethonin is inhibited by MDM2 in a dose dependent manner, MDM2 N-terminus interacts with telethonin and redirects it to the nucleus. In cells MDM2 is involved in the regulation of proteasomal turnover of telethonin. [5]
One interaction that is associated with pathology is with bone morphogenetic protein-10 (BMP10). The interaction between telethonin and BMP10 is thought to act as a sensor of increased wall stress of the left ventricle. A BMP10 variant is associated with hypertension dilated cardiomyopathy. In this case, BMP10 binding to telethonin is reduced, and its extracellular secretion is increased, causing cardiomyocyte hypertrophy. [16]
Yeast two hybrid screens (Y2H) of skeletal muscle cDNA libraries with baits for the E3 ubiquitin ligases MURF1 and MURF2 have shown an interaction with telethonin, suggesting MURF1/2 may target telethonin for degradation by the proteasome. [17] An interaction between the pro-apototic protein Siva and Telethonin has also shown by Y2H, and verified by ‘’in-vitro’’ pull-down assays, and immunoflurescence experiments showing a colocalization of both proteins in transfected HEK293 cells, but not yet confirmed ‘’in-vivo’’. [18]
Finally, the catalyitic domain of Protein Kinase D (PKD) has been shown to interact with telethonin. Telethonin has the PKD recognition motif Arg-X-X-Ser, suggesting PKD may regulate sarcomeric assembly and turnover through phosphorylation of telethonin. [19]
Pathologies associated with Telethonin
Different mutations in Telethonin have been associated with several myopathies. Mutations can lead to limb-girdle muscular dystrophy type 2G (LGMD2G) [20], to hypertrophic cardiopathy, [21] and dilated cardiomyopathy.
Two mutations found in the Tcap gene causing deletion of the telethonin C-terminal region and lost of the titin kinase phosphorylation site [20], were reported in Brazilian patients with LGMD2G.
Defects in the MLP-telethonin association have been linked to human dilated cardiomyopathy and heart failure (Knöll 2002). Mutations that affect ability of MLP to interact with telethonin resulting in the loss of telethonin binding facilitate its mislocalization from the complex with titin, and lead to defects in the Z-disk and progression of dilated cardiomyopathy. Knöll et al. conclude that genetic mutations causing a incorrect interaction between telethonin and MLP can lead to a development of human dilated cardiomyopathy through modifications in the conformation and function of titin. [21]
Finally, decreased staining for telethonin in type II fibers, and in early stages of fiber atrophy were reported in 10 cases of neurogenic atrophy, [22] indicating a selective downregulation of telethonin in these cases. These observations can be corelated to ‘’in-vivo’’ studies of short-term dennervation (two days) in rat skeletal muscle, in which ‘’Tcap’’ mRNA was reduced by about 50%. [3].
References
- ↑ Zhang S, Londhe P, Zhang M, Davie JK. Transcriptional analysis of the titin cap gene. Mol Genet Genomics. 2011 Mar;285(3):261-72. Epub 2011 Feb 9. PMID:21305318 doi:10.1007/s00438-011-0603-6
- ↑ Valle G, Faulkner G, De Antoni A, Pacchioni B, Pallavicini A, Pandolfo D, Tiso N, Toppo S, Trevisan S, Lanfranchi G. Telethonin, a novel sarcomeric protein of heart and skeletal muscle. FEBS Lett. 1997 Sep 29;415(2):163-8. PMID:9350988
- ↑ 3.0 3.1 Tian LF, Li HY, Jin BF, Pan X, Man JH, Zhang PJ, Li WH, Liang B, Liu H, Zhao J, Gong WL, Zhou T, Zhang XM. MDM2 interacts with and downregulates a sarcomeric protein, TCAP. Biochem Biophys Res Commun. 2006 Jun 23;345(1):355-61. Epub 2006 May 2. PMID:16678796 doi:10.1016/j.bbrc.2006.04.108
- ↑ Vainzof M, Moreira ES, Suzuki OT, Faulkner G, Valle G, Beggs AH, Carpen O, Ribeiro AF, Zanoteli E, Gurgel-Gianneti J, Tsanaclis AM, Silva HC, Passos-Bueno MR, Zatz M. Telethonin protein expression in neuromuscular disorders. Biochim Biophys Acta. 2002 Oct 9;1588(1):33-40. PMID:12379311
- ↑ 5.0 5.1 Tian LF, Li HY, Jin BF, Pan X, Man JH, Zhang PJ, Li WH, Liang B, Liu H, Zhao J, Gong WL, Zhou T, Zhang XM. MDM2 interacts with and downregulates a sarcomeric protein, TCAP. Biochem Biophys Res Commun. 2006 Jun 23;345(1):355-61. Epub 2006 May 2. PMID:16678796 doi:10.1016/j.bbrc.2006.04.108
- ↑ 6.0 6.1 Zou P, Pinotsis N, Lange S, Song YH, Popov A, Mavridis I, Mayans OM, Gautel M, Wilmanns M. Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Nature. 2006 Jan 12;439(7073):229-33. PMID:16407954 doi:10.1038/nature04343
- ↑ Zou P, Gautel M, Geerlof A, Wilmanns M, Koch MH, Svergun DI. Solution scattering suggests cross-linking function of telethonin in the complex with titin. J Biol Chem. 2003 Jan 24;278(4):2636-44. Epub 2002 Nov 20. PMID:12446666 doi:10.1074/jbc.M210217200
- ↑ Pinotsis N, Petoukhov M, Lange S, Svergun D, Zou P, Gautel M, Wilmanns M. Evidence for a dimeric assembly of two titin/telethonin complexes induced by the telethonin C-terminus. J Struct Biol. 2006 Aug;155(2):239-50. Epub 2006 Apr 27. PMID:16713295 doi:10.1016/j.jsb.2006.03.028
- ↑ 9.0 9.1 Mayans O, van der Ven PF, Wilm M, Mues A, Young P, Furst DO, Wilmanns M, Gautel M. Structural basis for activation of the titin kinase domain during myofibrillogenesis. Nature. 1998 Oct 29;395(6705):863-9. PMID:9804419 doi:10.1038/27603
- ↑ 10.0 10.1 Furukawa T, Ono Y, Tsuchiya H, Katayama Y, Bang ML, Labeit D, Labeit S, Inagaki N, Gregorio CC. Specific interaction of the potassium channel beta-subunit minK with the sarcomeric protein T-cap suggests a T-tubule-myofibril linking system. J Mol Biol. 2001 Nov 2;313(4):775-84. PMID:11697903 doi:10.1006/jmbi.2001.5053
- ↑ Kontrogianni-Konstantopoulos A, Bloch RJ. The hydrophilic domain of small ankyrin-1 interacts with the two N-terminal immunoglobulin domains of titin. J Biol Chem. 2003 Feb 7;278(6):3985-91. Epub 2002 Nov 19. PMID:12444090 doi:10.1074/jbc.M209012200
- ↑ 12.0 12.1 Frey N, Olson EN. Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins. J Biol Chem. 2002 Apr 19;277(16):13998-4004. Epub 2002 Feb 12. PMID:11842093 doi:10.1074/jbc.M200712200
- ↑ 13.0 13.1 Kojic S, Medeot E, Guccione E, Krmac H, Zara I, Martinelli V, Valle G, Faulkner G. The Ankrd2 protein, a link between the sarcomere and the nucleus in skeletal muscle. J Mol Biol. 2004 May 28;339(2):313-25. PMID:15136035 doi:10.1016/j.jmb.2004.03.071
- ↑ Markert CD, Ning J, Staley JT, Heinzke L, Childers CK, Ferreira JA, Brown M, Stoker A, Okamura C, Childers MK. TCAP knockdown by RNA interference inhibits myoblast differentiation in cultured skeletal muscle cells. Neuromuscul Disord. 2008 May;18(5):413-22. Epub 2008 Apr 28. PMID:18440815 doi:10.1016/j.nmd.2008.03.010
- ↑ Nicholas G, Thomas M, Langley B, Somers W, Patel K, Kemp CF, Sharma M, Kambadur R. Titin-cap associates with, and regulates secretion of, Myostatin. J Cell Physiol. 2002 Oct;193(1):120-31. PMID:12209887 doi:10.1002/jcp.10158
- ↑ Nakano N, Hori H, Abe M, Shibata H, Arimura T, Sasaoka T, Sawabe M, Chida K, Arai T, Nakahara K, Kubo T, Sugimoto K, Katsuya T, Ogihara T, Doi Y, Izumi T, Kimura A. Interaction of BMP10 with Tcap may modulate the course of hypertensive cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2007 Dec;293(6):H3396-403. Epub 2007 Oct, 5. PMID:17921333 doi:10.1152/ajpheart.00311.2007
- ↑ Witt SH, Granzier H, Witt CC, Labeit S. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol. 2005 Jul 22;350(4):713-22. PMID:15967462 doi:S0022-2836(05)00552-8
- ↑ Mihatsch K, Nestler M, Saluz HP, Henke A, Munder T. Proapoptotic protein Siva binds to the muscle protein telethonin in cardiomyocytes during coxsackieviral infection. Cardiovasc Res. 2009 Jan 1;81(1):108-15. Epub 2008 Oct 11. PMID:18849585 doi:10.1093/cvr/cvn276
- ↑ . PMID:155114163
- ↑ 20.0 20.1 Moreira ES, Wiltshire TJ, Faulkner G, Nilforoushan A, Vainzof M, Suzuki OT, Valle G, Reeves R, Zatz M, Passos-Bueno MR, Jenne DE. Limb-girdle muscular dystrophy type 2G is caused by mutations in the gene encoding the sarcomeric protein telethonin. Nat Genet. 2000 Feb;24(2):163-6. PMID:10655062 doi:10.1038/72822
- ↑ 21.0 21.1 Knoll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML, Hayashi T, Shiga N, Yasukawa H, Schaper W, McKenna W, Yokoyama M, Schork NJ, Omens JH, McCulloch AD, Kimura A, Gregorio CC, Poller W, Schaper J, Schultheiss HP, Chien KR. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002 Dec 27;111(7):943-55. PMID:12507422
- ↑ Schroder R, Reimann J, Iakovenko A, Mues A, Bonnemann CG, Matten J, Gautel M. Early and selective disappearance of telethonin protein from the sarcomere in neurogenic atrophy. J Muscle Res Cell Motil. 2001;22(3):259-64. PMID:11763198