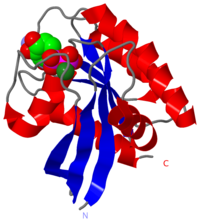
H-RasK117R mutant (2quz) in Costello Syndrome
(see also GTPase_HRas)
HRAS is a protein involved in the regulating cell division after a stimulation about growth factor. This protein belongs to a subfamily called Ras.
This protein is an enzyme which is mutated in a genetic disease called Costello syndrome and in cancers like bladder cancer. 85 to 90% of patients have got mutations in glycine 12 and 13. The mutations of HRAS Lys117Arg was detected in very few patients. They pronounce less palmar and plantar creases and present autistic behaviour. But they have similar features than other patients.
Structure of HRAS
2QUZ is an A chain linear structure of sequence from Homo sapiens. The gene encoding this protein is on the chromosome 11. This protein presents 166 amino acids. Its registry number is EC 3.6.5.2.
-EC 3 : This enzymes is a hydrolase.
-EC 3.6 : This enzyme acts on acid anhydrides.
-EC 3.6.5 : This enzyme acts on GTP; involved in cellular and subcellular movement.
-EC 3.6.5.2 : This enzymes is a small monomeric GTPase.
HRAS protein is a GTPase. It acts like a signaling protein. Its structure is discovered by X-Ray Diffraction.
Peptide sequence
1 mteyklvvvg aggvgksalt iqliqnhfvd eydptiedsy rkqvvidget clldildtag
61 qeeysamrdq ymrtgegflc vfainntksf edihqyreqi krvkdsddvp mvlvgnkcdl
121 aartvesrqa qdlarsygip yietsaktrq gvedafytlv reirqh
The arginine takes the place of the lysine 117 in HRAS Lys117Arg.
Conformation molecular and Hydrolysis
HRAS has got 5 helix and 6 strands. It presents sites of interactions with GTP, GDP, GEF, magnesium ion and other effectors like GAP. This enzyme presents an isoprenyl group on its C-terminus.
HRAS hydrolyses GTP to GDP at the level of fixation site. The side chain Y32 is very displaced at the level of the commutator 1. It travels the space between the loop 2 and the rest of the protein instead of being directed outwards. This side chain Y32 movement involves hydrolysis of GTP to GDP. It is a conformation change.
HRAS catalyses the reaction : GTP + H2O = GDP + Phosphate.
One hand, nucleophilic attack is facilitated by low-energy interaction between Thr35, Gly60 and Lys16 which play the role of acceptors and the phosphate group γ which plays the role of donor.
Other hand, the hydrogen bond between the Gly13 ( donor ) and the oxygen of the bridge βγ stabilizes the movement of the charges.
Function in the cell
Thanks to the presence of isoprenyl group on its C-terminus, HRAS is associated with the cell membrane.
HRAS protein acts as a molecular on/off switch due to the conformation change. The protein is activated when it has a GTP-bound conformation and vice versa. It activates proteins which are required for the propagation of the signal as RAF kinase. This enzymes phosphorylates and activates MEK. The reactions continue. HRAS is an early enzyme in growth signal transduction.
HRAS is inactive and is bound to GDP. Thanks to the action of GEF stimulated by the signal, GDP is released and is replaced by a GTP. HRAS is active and hydrolyse GTP activating downstream proteins. The rate of hydrolysis is increasing by a protein of GAP class. HRAS returns to its original conformation ( inactive conformation ).
You can see an animation about the function.
Mutation of HRAS in Costello syndrome
Costello syndrome is a mental retardation syndrome discovered by Costello in 1971 and 1977. Children who are affected by this disease suffer from many problems as postnatal growth retardation or tumor predisposition.
The mutation affects the codon 117 : Lysine is replaced by Arginine.
HRAS mutation alters interaction between the side chain and causes unfavorable nucleotide binding properties. The substitution causes additional polar interaction with residues of the nucleotide binding site even if the two amino acids belongs to the same class (basic amino acid). Side chain of the lysine has got a dual character : polar and nonpolar. Side chain of the arginine presents a guanidinium group which creates an hydrogen bond with Asparagine85. The lysine stabilizes nucleotide binding by ites aliphatic interaction with the base and its amino group interacts with ribose oxygen O4 and with a segment of main chain loop ( Gly13, CO ). The rearrangements introduce destabilizing of nucleotide binding by reducing binding affinities.
After experiments, the speed of hydrolysis is increased. That is the result in constitutive activation of HRAS and effectors which are activated by activiting HRAS. Indeed, levels of phosphorylated effectors in the cascade and HRAS are elevated compared to cells without mutation Lys117Arg. HRAS is activated even in the abscence of stimulating signal. GTPase activity is not affected and the enzyme responds normally to the linking of GAP.
Inactive HRAS is bound with GDP but the affinty is low. So the protein releases GDP. The concentration of GTP is more elevated than the concentration of GDP, the enzyme binds to GTP quickly. As the affinty of the bound is low, HRAS hydrolyses quickly GTP to GDP and the cycle continues. HRAS stays inactive during a short time.