Hint auto-proteolytic protein-processing domains
From Proteopedia
Hint auto-proteolytic protein-processing domains
Hint (Hog/ INTein) domain superfamily includes four types of auto-catalytic domains. These domains process the proteins in which they are present by protein-splicing, self-cleavage and ligation activities:
1. Inteins
2. Hog Hint domains
3. A-type Bacterial intein-like (BIL) domains
4. B-type BIL domains
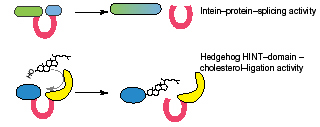
 All Hint domain types are 130 to 160 amino acid long, sharing 4-6 [conserved sequence motifs. Intein and Hog Hint domain families also share the same structural fold and active site arrangement. BIL domains probably have this fold as well. Hint domains have similar biochemical reactions, by which they remove themselves from their protein precursors.
All Hint domain types are 130 to 160 amino acid long, sharing 4-6 [conserved sequence motifs. Intein and Hog Hint domain families also share the same structural fold and active site arrangement. BIL domains probably have this fold as well. Hint domains have similar biochemical reactions, by which they remove themselves from their protein precursors.
Intein domains are selfish genetic elements, embedded in-frame within highly conserved regions of essential proteins in species from the three domains of life. The intein domain is translated with its protein host, and excises itself out by efficient protein-splicing of its two flanks with a peptide bond. Protein-splicing depends on conserved active-site residues within the intein, and on an invariable Cys, Ser or Thr nucleophilic C'-flanking residue following the intein domain.
Hog autocatalytic domains are found in animal Hedgehog proteins. The Hint domain is followed by a cholesterol-binding domain, and autocatalyses its cleavage from the N' Hedge domain by attaching a cholesterol molecule to it. The released Hedge domain is further modified and secreted from the cell as a developmental signal.
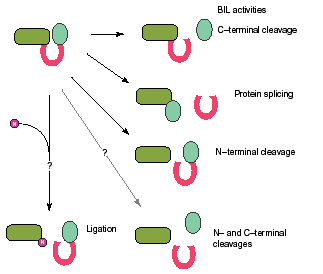
Bacterial Intein-Like Domains (BILs) are ~140 amino acids long, and have characteristic sequence motifs of the Hint domain. These domains are highly modular, being integrated in variable positions of non-conserved protein hosts, some of which are extracellular. Similarly to other types of Hint domains, BIL domains may contribute to their host variability through several post-translational biological activities like protein-splicing, self-cleavage and ligation. BILs may also be involved in maturation and conjugation of poly-Ubiqutin-like precursor proteins.
Related links:
Inteins- protein introns, Weizmann Institute of Science
InBase: The New England Biolabs Intein Database
--Bareket.dassa 11:27, 29 November 2007 (IST)
