Image:Crypticrepeats.jpg
From Proteopedia
No higher resolution available.
Crypticrepeats.jpg (599 × 296 pixel, file size: 39 KB, MIME type: image/jpeg)
Summary
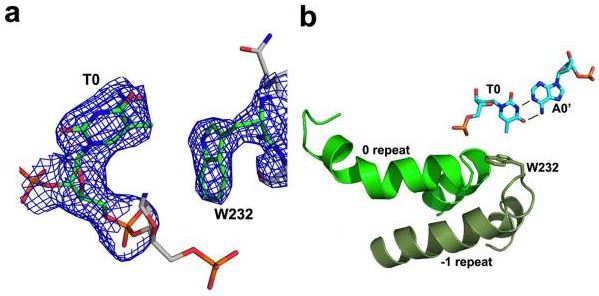
2Fo-Fc electron density maps contoured around thymine at position ‘0’ and tryptophan 232 in the ‘−1’ repeat. b: Residues 221 to 239 and residues 256 to 273 each form a helix and an adjoining loop that resembles helix 1 and the RVD loop in the canonical repeats; the remaining residues in each region are poorly ordered. W232 forms a non polar van der Waals contact with the methyl carbon of the thymine base at position 0.
Licensing
{{subst:No license from license selector|Don't know}}
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | User | Dimensions | File size | Comment | |
|---|---|---|---|---|---|
| (current) | 10:58, 3 January 2013 | Philipp Warmer (Talk | contribs) | 599×296 | 39 KB | 2Fo-Fc electron density maps contoured around thymine at position ‘0’ and tryptophan 232 in the ‘−1’ repeat. b: Residues 221 to 239 and residues 256 to 273 each form a helix and an adjoining loop that resembles helix 1 and the RVD loop in the ca |
- Edit this file using an external application
See the setup instructions for more information.
Links
The following pages link to this file:
Metadata
This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original state, some details may not fully reflect the modified image.
| Date and time of data generation | 10:46, 3 January 2013 |
|---|---|
| Author | Philipp |
| Date and time of digitizing | 10:46, 3 January 2013 |
| DateTimeOriginal subseconds | 91 |
| DateTimeDigitized subseconds | 91 |
