Trypsin is a serine protease that found in the digestive system of many vertebrates, where it hydrolyses proteins[1].
Trypsin is produced in the pancreas as the inactive zymogen protease called trypsinogen. trypsinogen secreted into the small intestine where it activates trypsinogen into trypsin by the enzyme Antrookinaz using proteolytic decomposition.
Trypsin Break peptide chains at the carboxyl side of the amino acids lysine or arginine,
except when it is followed by proline. .
Function
Trypsin catalyzes the hydrolysis of peptide bonds and cutting proteins into smaller peptides in the duodenum.
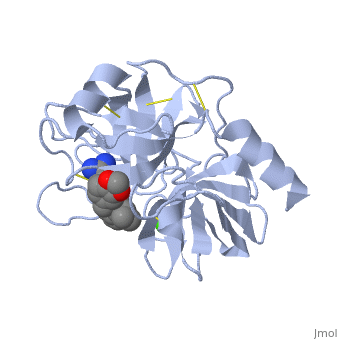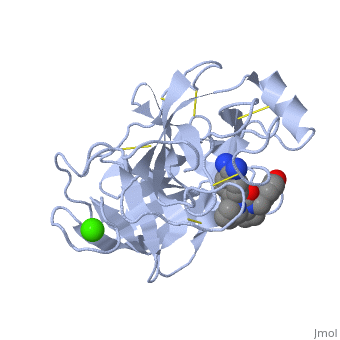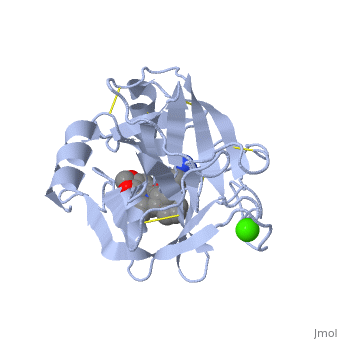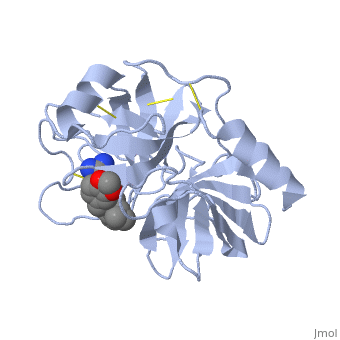
Structure and Mechanism
Trypsin is a medium size globular protein that contains either and .
The enzymatic mechanism of Trypsin is similar to the other serine proteases. These enzymes contain a catalytic triad consisting of , These three residues makes the active site serine nucleophilic [2].
Trypsin contains an "oxyanion hole" formed by the backbone amide hydrogen atoms of , which serves to stabilize the developing negative charge on the carbonyl oxygen atom of the cleaved amides.
The located in the catalytic pocket of trypsin is responsible for attracting and stabilizing positively charged lysine or arginine, and as a result responsible for the specificity of the enzyme [3].
Structural highlights
There are different ways to present the protein structure :, and . Another way to view the protein is that show the protein from the N (blue) to C terminal (green).